Biomedical Engineering Reference
In-Depth Information
but harbors the most aggressive and resistant cells. On reaching the targeted cells
the nanocarrier must become ''sticky'' or ''cell binding'' to interact with the tumor
cell for efficient cellular uptake. A nanocarrier capable of simultaneously
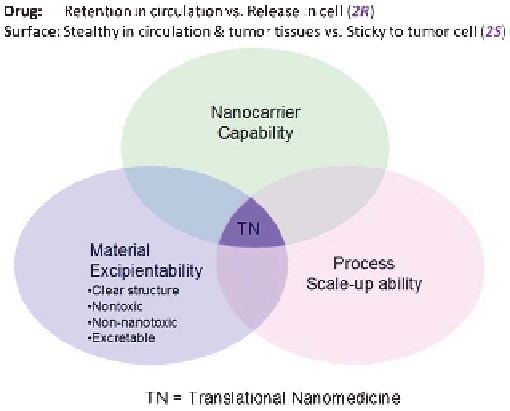
satisfying such opposite 2R2S requirements at the right time and the right place,
that is, ''drug Retention in blood circulation vs. Release in tumor cells (2R)'' and
''surface Stealthy in blood circulation and tumor tissues vs. Sticky to tumor cells
(2S)'', will deliver the drug specifically to the tumor cells, giving rise to high
therapeutic efficacy and few side effects.
While the 2R2S capability of a nanocarrier may render the resulting
nanomedicine efficacious and potentially safe for clinical translation, two other
elements, namely the feasibility of the nanocarrier materials to be proved for use
as excipients (referred to as material excipientability) and the ability to establish
scaled-up production processes for good manufacturing practice (GMP) for the
nanocarrier and its formulation with the drug (nanomedicine) (referred to as
process scale-up ability) are also indispensible for the nanomedicine to be truly
translational from the benchtop to the bedside (Figure 3.2).
8
Most of our current
research is focused on using new material design and chemistry to improve the
2R2S capability; however, research aimed at translational applications should
comprehensively consider the other two elements at an early stage.
Herein, we briefly review the approaches addressing nanocarriers' 2R2S
capability and summarize the factors affecting material excipientability and
process
d
n
4
y
3
n
g
|
2
scale-up
ability,
aimed
at
promoting
the
developments
of
truly
translational nanomedicine for cancer drug delivery.
Figure 3.2
The three elements for translational nanomedicine: the nanocarrier
should have the 2R2S capability and its material should be suitable for
excipient use (referred to as material excipientability); the production of
the nanocarrier and its formulation with drug (nanomedicine) should be
able to scale up for good manufacture process (GMP) (scale-up ability).
Reprinted with permission from ref. 8. Copyright 2012 Elsevier.