Biomedical Engineering Reference
In-Depth Information
micelles (at this concentration), but the CPs in dimethyl ether are substantially
lower than those in trifluoromethane. Hexafluoroethane was found too weak
to dissolve the copolymer. While tetrafluoroethane dissolved the copolymer, it
did not produce micelles.
These two figures are useful to approximate the solvent capacity and
selectivity, which are usually defined in terms of mole or weight fractions.
Capacity is a measure of the affinity of the solvent for the polymer. For a given
solvent, the capacity roughly scales with density and hence increases with
increasing pressure. Therefore, in comparing two different solvents, the one
with a lower CP for a given polymer is deemed to have a higher capacity. For
example, considering the difference in CP, dimethyl ether should have a higher
capacity for the diblock than trifluoromethane due to a much lower CP.
Selectivity is a measure of the affinity of a given solvent for one polymer over
another. So, for the same solvent, a large difference in CP for two polymers
means a high selectivity. Therefore, when the polymers are joined in a
d
n
4
y
3
n
g
|
1
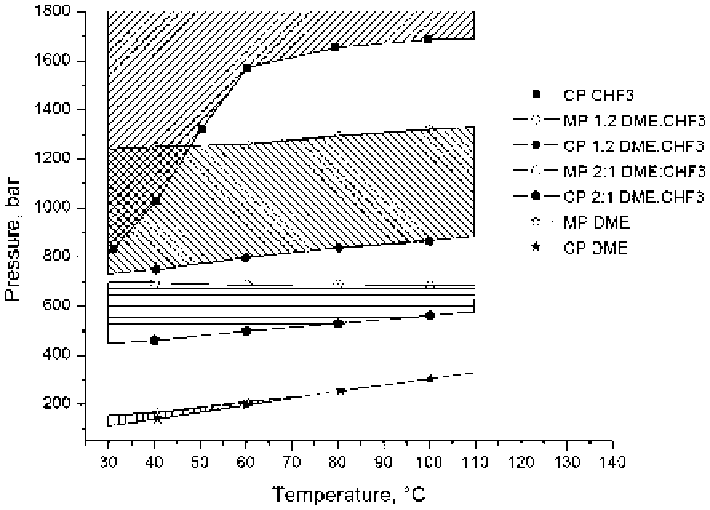
Figure 13.13
Pressure-temperature phase diagram of PEG-b-PCL (5k-b-11k) in
near-critical trifluoromethane, dimethyl ether, and mixtures. Micellar
regions are the shaded regions between the CP and MP curves. Note:
the upper limit of the shaded region for pure trifluoromethane is the
experimental boundary, not the MP. The concentration of polymer in
solution was 1 wt% for all but the pure dimethyl ether, which was
2 wt%. (Reproduced from Green et al.
19
with permission from the
American Chemical Society.)