Biomedical Engineering Reference
In-Depth Information
therapeutic efficacy and minimal toxicity would be the one that is stable in the
blood compartments but labile in cancer cells.
The linker should first be stable in blood circulation to ensure low toxicity.
8
For example, the PK1 conjugate in which DOX was covalently bound to
poly[N-(2-hydroxypropyl)methacrylamide] (PHPMA) via a blood-stable but
lysosome-labile peptidyl linker had very low dose-limiting toxicity.
9
In phase I
clinical and pharmacokinetic studies, PK1 had a maximum tolerated dose
(MTD) of 320 mg m
22
, and no congestive cardiac failure despite individual
cumulative doses up to 1,680 mg m
22
.
9
However, both the PHPMA-
PTX prodrug named PNU166945
10
and the poly(methacryloylglycinamide)
(PMAG)-CPT prodrug
11
experienced dose-limiting toxicity in phase I clinical
study because their easily hydrolysable ester linkers released the drugs while in
circulation.
12
Upon reaching the intracellular target, the prodrug must efficiently release
the parent drug to exert its pharmaceutical action because only the liberated
drug becomes active.
13
Stable prodrugs, e.g. drugs bonded to poly(lactic-co-
glycolic acid) (PLGA)
14
or poly(
L
-aspartic acid) [P(Asp)],
15
showed low or
even no anticancer activity. It is preferable that the linker be cleavable in the
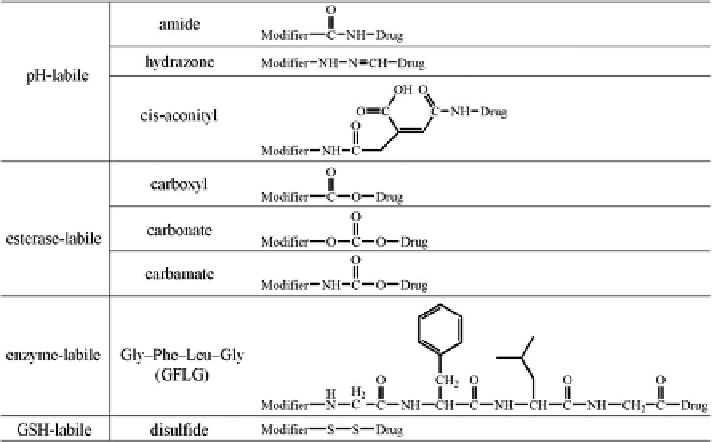
tumor microenvironment. This is achieved by using labile linkers responsive to
the tumor's extracellular or intracellular stimuli (Figure 11.2).
16
Lysosomal
pH-labile linkers (e.g., amide, hydrazone, or cis-aconityl bonds) ensure the
intracellular drug release in lysosomes and are widely used.
17
For instance,
DOX was conjugated to the P(Asp) block of its block copolymer poly(ethylene
glycol) (PEG)-b-P(Asp) via acid-labile amide
18
d
n
4
y
3
n
g
|
5
or hydrazone linkers.
19
DOX
Figure 11.2
Commonly used extracellular and intracellular stimuli-labile linkers in
prodrugs.