Biomedical Engineering Reference
In-Depth Information
also constructed with different polymer compositions.
18
These new complex
micelles with controllable channels may be promising candidates for use in
controlled uptake/release processes.
In comparison with typical core-shell micelles as drug carriers, besides a
well-defined core-shell structure, a nanoscale size (10-100 nm), the ability to
solubilize water-insoluble drugs, and prolonged blood circulation times,
19
CMSCs also have other advantages, including facile manipulation of the drug
release rate, efficient prevention of burst drug release, and degradation of the
micelle core, due to their unique structures. CMSCs are promising
nanocarriers for drug delivery due to their unique structure of an associated
core as a drug reservoir, a collapsed inner shell as a barrier for burst drug
release and enzyme invasion, and a soluble corona both as a stabilizer for the
nanoparticles and channels for mass exchange between the core and the outer
milieu.
20
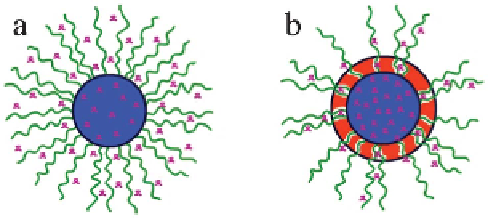
As indicated in Figure 9.12, drug release from simple core-shell
micelles (Figure 9.12a) may occur in all directions and burst release is often
observed. For CMSCs (Figure 9.12b), drug release is expected mainly through
the channels due to the protection of the shell layer of the PNIPAM, thus
effectively reducing the risk of burst release. At the same time, the PNIPAM
shell can also protect the micelle core and drug from invasion by enzymes,
which enables the CMSCs to be used as nanocarriers for oral drug delivery.
Several efforts have been made on the application of CMSCs in controlled
drug release.
Shi et al.
21
have prepared complex micelles with a common PLA core and a
mixed PEG/PNIPAM shell by simultaneous micellization of the diblock
copolymers poly(
L
-lactide)-b-poly(N-isopropylacrylamide) (PLA-b-PNIPAM)
and poly(ethylene glycol)-b-poly(
L
-lactide) (PEG-b-PLA) in aqueous solution
at room temperature, as illustrated in Figure 9.13. Upon increasing the
temperature above the LCST of PNIPAM, these complex micelles could be
converted into a CSC structure composed of a PLA core, a collapsed PNIPAM
shell, and a soluble PEG corona. The PEG chains stretched from the PLA core
and penetrated through the PNIPAM shell to the outer milieu, leading to the
formation of PEG channels. The PNIPAM block could collapse on the PLA
core surface and the density of the PEG channels decreased on increasing the
d
n
4
y
3
n
g
|
7
Figure 9.12
Schematic illustration of drug release from (a) core-shell micelles and (b)
CMSC micelles with surface channels.
20