Biomedical Engineering Reference
In-Depth Information
d
n
4
y
3
n
g
|
8
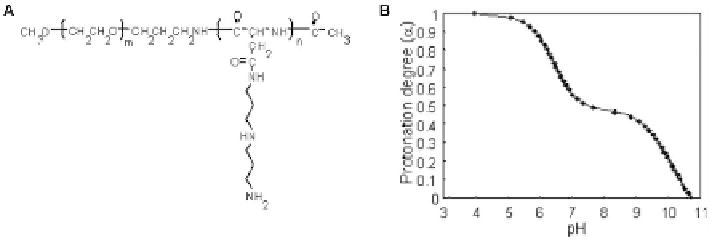
Figure 7.6
(A) Chemical structure of PEG-DPT. (B) Change in protonation degree
(a) with pH for Boc-Asp(DPT)-Pr. (Adapted from Itaka et al.
90
with
permission from the American Chemical Society.)
directing to the enhanced intracellular activity of siRNA through the buffering
capacity in the endosomal compartment. Notably, the gene knockdown
abilities of the siRNA/PEG-DPT complex were remarkable, especially at N/P
$ 10: it showed more than an 80% knockdown, which exceeded commercially
available RNAiFect. Meanwhile, The siRNA/PEG-DPT complexes showed
comparable abilities of gene knockdown, even after co-incubation with serum
for 30 min, which indicated the excellent feasibility of the PEG-DPT/siRNA
complex, particularly under physiological conditions.
90
In a separate study, the same group constructed pH-sensitive and targetable
polyion complex (PIC) micelles by electrostatic assembly of poly(
L
-lysine) and
lactosylated PEG-siRNA conjugates with acid-labile linkages (Lac-PEG-
siRNA), which exhibited significant gene silencing for firefly luciferase
expression in HuH-7 hepatoma cells. The PIC micelles achieved far more
effective RNAi activity than the Lac-PEG-siRNA conjugate alone, viz. the
50% inhibitory concentration (IC
50
) was found to be 1.3 nM and 91.4 nM for
the PIC micelle and Lac-PEG-siRNA conjugate, respectively. There was
almost a 100-fold increase in RNAi activity with the PIC micelles. Several
important factors were likely to be synergistically involved in the pronounced
RNAi activity of the PIC micelles, such as improved stability against
enzymatic degradation, minimal interaction with serum proteins, enhancement
of cellular uptake through asialoglycoprotein (ASGP) receptor-mediated
endocytosis, and the effective transport of free siRNA from endosomes into
the cytoplasm due to the cleavage of the acid-labile linkage allowing the release
of hundreds of free PEG strands to increase the colloidal osmotic pressure.
91
Convertine et al. synthesized a family of diblock copolymer siRNA carriers
using controlled reversible addition-fragmentation chain transfer (RAFT)
polymerization. The carriers were composed of a positively charged block of
dimethylaminoethyl methacrylate (DMAEMA) to mediate siRNA binding
and a second pH-responsive endosome-releasing block composed of
DMAEMA and propylacrylic acid (PAA) in roughly equimolar ratios along
with butyl methacrylate (BMA). However, the polymers could not form