Biomedical Engineering Reference
In-Depth Information
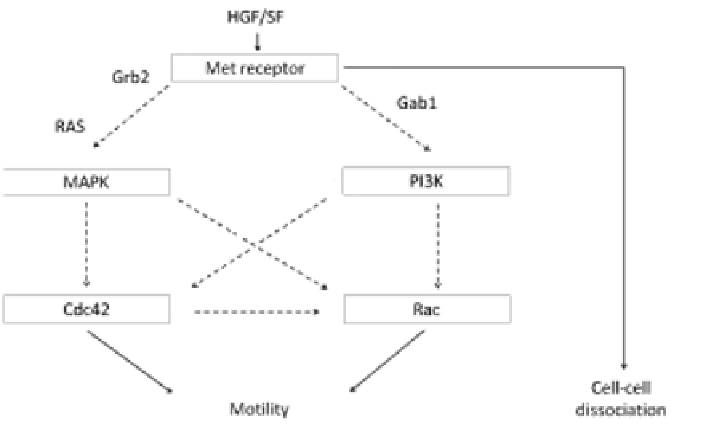
FIGURE 5.1: Simplified schematic representation of HGF/SF signaling cas-
cades in the control of ARO cell motogenesis. Met receptors activate a se-
ries of intracellular events that induce the recruitment of phosphatidylinositol
3-kinase (PI3K) and mitogen-activated protein kinase (MAPK). Both intra-
cellular messengers are able to activate Rac and Cdc42 molecules. Increases
in Rac and Cdc42 activity trigger cell motility. The dashed arrows stand for
indirect pathways not completely included in the model.
MAPK molecules then diffuse within the cell cytosol, where they induce the
production of Cdc42 and Rac small GTP-ases [101, 423], which in turn stimu-
late the migratory capacity of the AROs. In particular, Cdc42, which can lead
to the activation of Rac itself [18], is considered to be a central regulator of
cell protrusive activity [125, 329]. Rac is instead required for lamellipodia and
membrane rues [42]. Finally, both Cdc42 and Rac mediate actin polymeriza-
tion by activating actin-related proteins, as Arp2/3 complexes [184, 328, 329].
Figure 5.1 diagrammatically represents the key biochemical processes just de-
scribed.
The same phenomenon has already been modeled in Chapter 2. This choice
allows a useful investigation of how the proposed CPM developments are able
to affect and improve the representation of a given biological process. Indeed,
each ARO individual can now be characterized by its own biophysical prop-
erties, such as its motility, that are realistically regulated by the external
chemical stimuli via well-defined intracellular dynamics. The resulting model
is highly flexible and able to characterize the healing of the cell population in
different conditions consistently with the experimental counterparts, making
clear the relevance of various mechanisms involved in determining the motile
phenotype. Indeed, it describes the migratory phenotype of the whole cell







Search WWH ::

Custom Search