Biomedical Engineering Reference
In-Depth Information
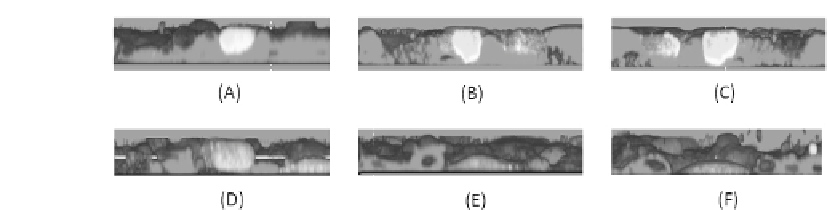
FIGURE 3.3: (See color insert.) Transmesothelial migration assay: nonma-
lignant transformed human pleural mesothelial cells (HPMCs), Met-5A cells
were labeled with the PKH26GL cell linker kit (colored in red), seeded on
coverslips coated with bronectin (10 g/mL), and grown until conuence
at 37
C. The human ovarian carcinoma cell line NIH:OVCAR-3 was stained
with 5 M CFSE 20 min at 37
C (colored in green). CFSE-labeled tumor
cells (1.5 10
5
) were plated on HPMCs and incubated for 3 h at 37
C. Non-
adherent OvCa were carefully removed. Samples were analyzed by sequential
scanning of the XY planes recorded along the Z-axis (step size: 1.5 m) and
then processed using the 3-dimensional reconstruction software bioView3D
and visualized as orthogonal views. In (A) a cell is on the top of the mesothe-
lial layer. In (B) and (C) cells are inducing opening in the layer and passing
through. In (D) a cell is in the middle of the layer. In (E) and (F) cells crossed
the mesothelial layer, and the cells rejoined closing the gaps previously created
over the malignant cells.
Ospedaliero-Universitaria S.Giovanni Battista-Molinette di Torino. The ex-
perimental model consists of plating an ovarian cancer cell line (NIH: OVCAR-
3) on a nonmalignant transformed human mesothelial cell layer (Met-5A),
grown on an extracellular matrix protein, as reproduced in Figure 3.3. Exper-
imental evidence then shows that single ovarian tumor cells' adhesion to the
monolayer is mainly mediated by the interactions between 1-integrin and
some of its epitopes [368] and selected ECM proteins (such as laminin, -
bronectin, vitronectin, and type I and IV collagen), which are secreted by the
mesothelial cells and form a sort of pericellular matrix around the layer. CD44-
hyaluronan and E-cadherins also interact with the mesothelial cells [234, 291].
The subsequent transmigration across the mesothelium requires instead
the activity of selected matrix metalloproteinases (MMPs), endopeptidases
that predominantly degrade any structural components of the ECM, along
with a variety of cell adhesion molecules [406]. They also affect the relative
cellular signaling pathways and functions and control cell migration. More-
over, the MMPs are involved in the release of cell-membrane-bound precur-
sors of many growth factors, whose receptors are MMP substrates, and are
able to cleave and activate their own zymogen forms [122]. In pathological
conditions, the MMPs actively contribute to cancer progression: clinical data
suggest that benign tumor cells acquire malignant properties following up-






Search WWH ::

Custom Search