Biomedical Engineering Reference
In-Depth Information
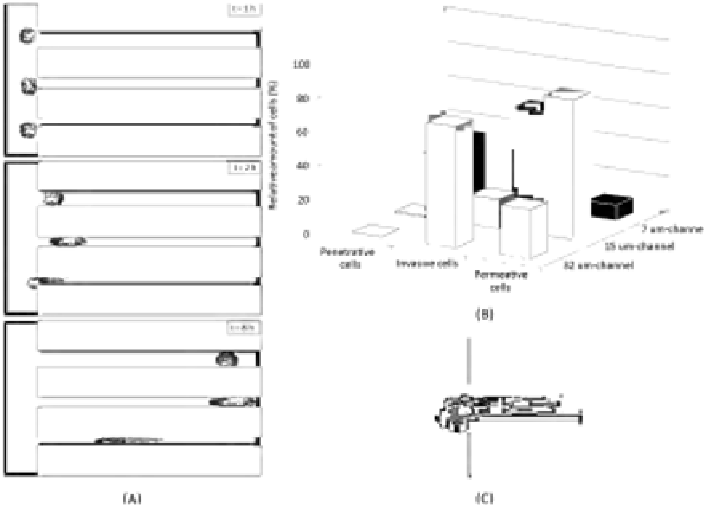
FIGURE 10.4: Migratory behavior within the microchannel structure of
cells with an elastic cytosol and a deformable nucleus (i.e.,
surface
;N
= 0:9).
(A) Images of a time-lapse simulation taken at t = 1 (top panel), 2 (middle
panel), and 8 (bottom panel) hours. The enhancement in nucleus elasticity
enables cells to enter also the smallest channel. For better visualization, in
the bottom panel, the nucleus is encircled manually. (B) Summary of cell mi-
gratory behavior within the matrix device. In the case of the widest channel,
cells display the same invasive behavior as in Figure 10.2. In the case of the
middle channels, the permeative phenotype is enforced. Finally, in the smaller
channel, cells acquire an invasive ability. The quantitative evaluation of spe-
cific cell motile phenotypes, represented in the histogram plot, is obtained by
performing 100 simulations. (C) Magnification of moving cell in close prox-
imity to the smaller channel entrance. It is obvious that the nucleus can now
squeeze to pass within the channel entrance, allowing the entire individual to
invade the structure.
nuclear envelope mediating its rigidity [144, 160]) or experimental treatments
with micromolar concentrations of bioactive lipid sphingosylphosphorylcholine
(SPC), whose activity leads to a substantial modification of the keratin net-
work toward a perinuclear rearrangement [28, 361].
The analysis of the model outcomes, summarized in Figure 10.4, reveals
that, in the case of the largest channel, the deformability of the nucleus does
not appreciably affect cell migratory behavior. When the channel dimension







Search WWH ::

Custom Search