Biomedical Engineering Reference
In-Depth Information
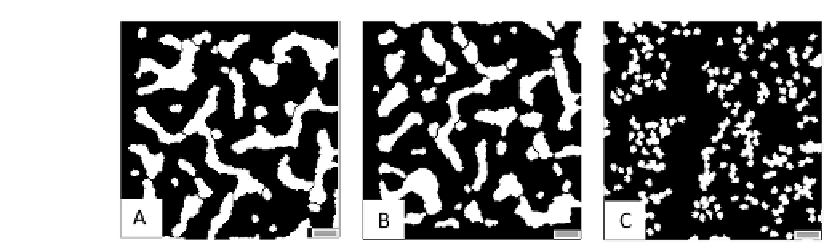
FIGURE 7.12: Partial disruption of TEC tubule formation by (A) inhibit-
ing cytoskeletal reorganization (with a high constant
perimeter
;C
for each in
Equation (6.4)), (B) interfering with the persistence component of cell mo-
tion (by setting
pers
= 0 for all cells in Equation (6.4)) and (C) disrupting
intercellular adhesion (with a high spatially homogeneous value of J
ext
E;E
). All
the other model parameters are the same as for the basic simulation in Figure
7.3. The scale bars are 100 m long.
in Equation (6.8)), we simulate the exclusion of arachidonic acid (respec-
tively, nitric oxide) biosynthesis, resembling cells pre-treated with widely used
PLA2 (respectively, eNOS) inhibitors (AACOCF3, respectively L-NAME or
L-NMMA [274, 284]). In both cases, VEGF-mediated intracellular calcium
events are not completely abolished and the relative microscopic mechanisms
(i.e., enhancement of cell adhesion, motility, and chemical sensitivity) still
occur, but with a significant delay and a lower intensity. Consequently, the
ultimate pattern morphologies feature an immature network shown in Figure
7.11, where several branches have partially formed, but have not been able
to organize in a single structure. In particular, the disruption of AA produc-
tion leads to lT
T
0.61L
T
(and thus pct = 0.39), while the disruption of NO
biosynthesis results in lT
T
0.73L
T
(pct = 0.27). This difference is caused by
the fact that AA partially regulates the recruitment of NO itself, see Equation
(6.8).
A potentially more ecient intervention strategy consists in blocking the
calcium-dependent cytoskeletal reorganization of TECs: in the model with a
high constant
perimeter
;C
for each and, experimentally, with phalloidin-like
compounds. The resulting capillary morphology, illustrated in Figure 7.12(A),
features in fact clumped, stunted and somewhat shorter and thicker sprouts,
as l
T
is 0.13L
T
and pct = 0.87. In particular, the vascular cords are 3{4
cells wide, with larger intervascular spaces. This phenomenology is consistent
with typical vascular hyperplasia [25] and is caused by the fact that TECs
are forced to keep their initial round morphology and, consequently, loose the
capacity to differentiate and polarize. Consequently, the TECs do not have
the persistent migration needed for the formation of a functional network, as
they can only form small, disconnected, branches along the gradients of VEGF
concentrations (see [192] for detailed comments).






Search WWH ::

Custom Search