Geology Reference
In-Depth Information
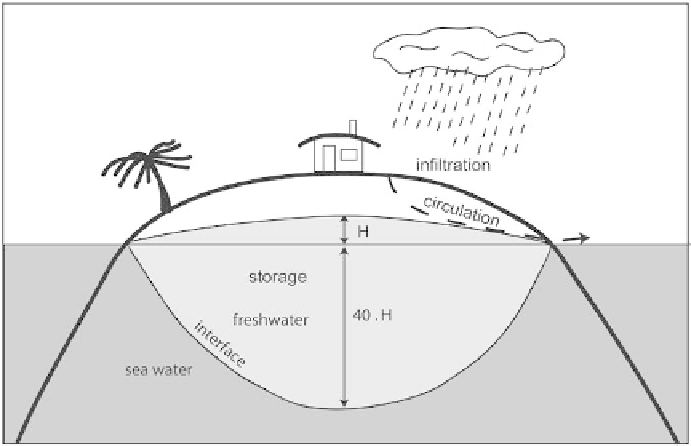
end of the 19th century by Ghyben and Herzberg. Fresh water, which is
less dense, fl oats on seawater with a sloping contact simplifi ed as a plane,
the interface, the position of which is dependent on the density difference
between the two environments, 1.000 for fresh water and approximately
1.025 for salt water (Figure 34). At equilibrium, the weight of the fresh
water column is equal to the weight of the salt water column. The
Ghyben-Herzberg law defi nes the position of the contact between the two
environments at equilibrium:
ρ
1
(P + H) = ρ
2
P or, approximately, P = 40 · H
where P is the depth to the interface; H is the hydraulic head (or the
piezometric level); ρ
1
is the density of fresh water; ρ
2
is the density of salt
water.
Figure
34
Theoretical geometry of the saltwater interface of an island aquifer.
In reality, mixing occurs through diffusion and the system is not entirely
static, as the aquifer drains into the sea, which is subject to tidal oscillations.
The two environments can therefore mix and form a layer of brackish water
of variable thickness. In addition, the anisotropy of the aquifer translates
into variations in the hydraulic gradient, which transforms the interface
into a complex surface with many lobes. The water table can also intersect
with the surface to form ponds or lagoons within which evaporation locally
lowers the piezometric surface, allowing the interface to rise.
Search WWH ::

Custom Search