Environmental Engineering Reference
In-Depth Information
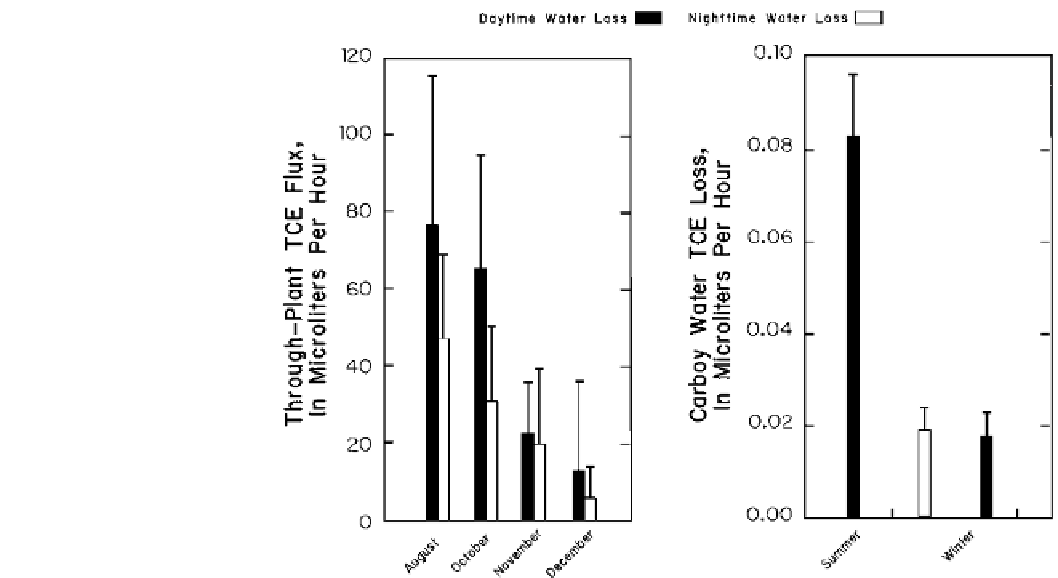
Fig. 13.16
The loss of TCE
added to plants under laboratory
conditions at different times of
the season as measured through
the plant and as loss from the
water solution. Loss occurred
even when transpiration was
decreased during no-leaf dormant
conditions.
remediation is an artifact of the evolutionary process that
offered a defensive advantage to such plants, because as we
saw earlier, some competing plants can release halogenated
organics. Rather than a reductive dechlorination reaction,
in which reduced organics are oxidized to provide a
source of electrons to reduce chlorinated compounds, the
dehalogenase directly oxidizes the TCE to CO
2
(Schnoor
et al. 1995).
In oxic groundwater, TCE can resist degradation, as
would be expected from its chemical structure. If plants
release exudates that support microbial activity and
the depression of DO levels, it may be possible for
methanogenic bacteria to predominate and release methane.
If oxic conditions exist in shallower parts of the aquifer, this
CH
4
can be oxidized by methanotrophic bacteria, which, in
turn, release methane monooxygenases (MMO), which also
can enzymatically degrade TCE. This process gratuitously
degrades TCE because the oxidation of methane requires a
MMO (Wilson and Wilson 1985). Whether or not this can be
considered phytoremediation is a matter
could be mineralized to CO
2
by bacteria in the root zone,
the question remained whether or not methanotrophic bacte-
ria were influencing TCE concentrations. Since these bacte-
ria can be found in the root zone, Brigmon et al. (1999)
investigated the interaction of these plant bacteria on the
TCE plume at the waste disposal site in South Carolina.
They reported the presence of these bacteria on the roots
and in the soil. However, at the site studied, up to 90% of the
TCE released was absorbed to the soils.
The fate of TCE in the unsaturated zone in vapor form
was studied by Narayanan et al. (1999). This pathway is
important in light of the high vapor pressure of TCE, and
because a fluctuating water-table level renders formerly
saturated TCE-contaminated sediments to be exposed to
air, and this will drive the gaseous diffusion of TCE into
the void spaces that can interact with plant roots. To test this
hypothesis of plant-root interaction with gaseous TCE, they
created in the laboratory an artificial aquifer to which sedi-
ment, water, TCE, and plants (alfalfa) were added. Samples
were collected periodically from this experimental water
table, unsaturated zone, and plant tissue. A vertical-upward
TCE gradient from the water table through the unsaturated
zone was detected, driven by diffusion from the concentra-
tion gradient (Fig.
13.17
). This is similar to that shown by
Lahvis et al. (1999) for the fate of MTBE that volatilized to
the vadose zone from gasoline-contaminated groundwater.
Vegetation lowered the transition zone between saturated
and unsaturated conditions, and caused the diffusive flux of
aqueous TCE in the water table to be upward into the
unsaturated zone. This presents a scenario where the plants
for debate,
however.
Brigmon et al. (1999) report the influence of the rhizo-
sphere on the fate of TCE at a waste disposal site in South
Carolina where TCE had been detected. The waste disposal
activities stopped in 1974, and the surficial fill material was
naturally populated over time by weeds to pine trees, specif-
ically loblolly pines (
Pinaceae spp
.). Anderson and Walton
(1995) had reported that the phenols released from pine trees
supported TCE mineralization in the rhizosphere relative to
unplanted soils. Whereas these studies showed that TCE
Search WWH ::

Custom Search