Environmental Engineering Reference
In-Depth Information
Fig. 13.13
At a UST release site in Beaufort, South Carolina, a
dissolved-phase plume of BTEX and MTBE compounds extended
downgradient in the surficial aquifer beneath a stand of 40-year-old
live oaks (
Quercus virginiana
). Monitoring wells are evident in front
and behind this particular tree (Photograph by author).
areas of a gasoline spill that contained 15% MTBE (in
such a mixture, MTBE has about 2,500 mg/L maximum
solubility in water). A mass balance indicated that most of
the MTBE was removed from the solution and found in the
air, and that MTBE detected in the plant had not been
transformed (Yu and Gu 2006).
These studies support the conclusion that in most cases,
MTBE is volatilized through the plant after uptake. Arnold
et al. (2007) investigated MTBE loss by existing vegetation
at a gasoline site in California characterized by MTBE- and
TBA-contaminated groundwater. The existing vegetation
consisted of mature conifers and was 33-43 ft (10-13 m)
tall. The depth to groundwater was not reported, although it
appeared to be between 5 and 15 ft (1.5-4.5 m) below
ground. In order to determine if the MTBE- and TBA-
contaminated groundwater beneath the conifers was being
taken up by these plants, leaf gas was collected and the
transpired moisture collected as a condensate in 125-mL
airtight plastic bags. These samples were then transferred
to standard 40-mL VOA vials and analyzed using standard
method EPA 8260B.
At a UST release site in Beaufort, South Carolina, a
dissolved-phase plume of BTEX and MTBE compounds
extended downgradient in the surficial aquifer beneath a
stand of live oak (
Quercus virginiana
) (Fig.
13.13
). The
depth to water table is on average about 13 ft (3.9 m) from
land surface, and the soils and sediments comprise sand
grains with very little (0.01%) natural organic matter
(Landmeyer et al. 2000). Precipitation at the site approaches
60 in./year (152 cm/year), as it is located near the Atlantic
Ocean. The live oak trees are at least 40 years old, and even
though they tend to have a predominantly shallow and
horizontal root structure, oak-tree roots were found at
depth near the water table in some monitoring wells near
Fig. 13.12
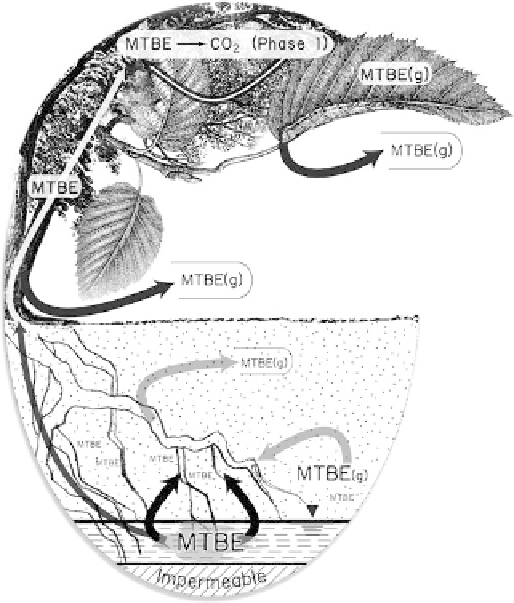
The potential fate of MTBE in the dissolved and gaseous
phases upon being taken up by a tree at a phytoremediation site. MTBE
(g) is the volatile phase.
MTBE taken up by transpiration was released unattenuated.
However, this measurement of MTBE loss is from stems
only that were monitored with the diffusion traps; the effect
of MTBE loss from leaves would increase this loss percent-
age. Much of the MTBE spiked into the solution that was not
volatilized remained in the roots and lower stems of the
cuttings. Such plant parts comprise the largest proportion
of biomass. Very little was found to be incorporated into
plant tissue, including the leaves.
Because the log
K
ow
of MTBE is about 1.2, it is not
surprising that many researchers have shown in the lab that
MTBE is transpired from solution with almost 100% recov-
ery in the air (Rubin and Ramaswami 2001; Hong et al.
2001). This could imply either dissolved-phase or gas-
phase uptake of MTBE from the subsurface (Fig.
13.12
).
Further evidence to indicate that MTBE in groundwater is
transpired to the atmosphere essentially intact and at near
100% efficiency was provided by Yu and Gu (2006). They
exposed weeping willow (
Salix babylonica
L.) cuttings
growing in hydroponic solutions in the laboratory to a
range of MTBE concentrations. The effect of MTBE con-
centration on the plant itself was measured by changes in
transpiration; only at MTBE concentrations near 400 mg/L
was a decrease in transpiration observed. Such high
concentrations would tend only to be found near source


Search WWH ::

Custom Search