Environmental Engineering Reference
In-Depth Information
such as formic and acetic acid. Although mineralization to
14
CO
2
was low (
0.17%), mineralization was greater (by
36%) in the presence of the organic acids.
Reilley et al. (1996) reported that for anthracene and
pyrene added to soils that were vegetated and unvegetated,
30-44% more degradation was observed when vegetation
was present. The work was carried out in the laboratory,
using fescue (
Festuca arundinacea
), alfalfa (
Medicago
sativa
) sundangrass (
Sorghum vulgare
), and switchgrass
(
Panicum virgatum
). Interestingly, the normal processes of
natural attenuation, such as leaching, abiotic degradation,
mineralization, and sorption were not significant factors in
the dissipation of the added PAHs. Moreover, total accumu-
lation of PAHs in the plant was less than 0.03% of that
initially added to the soil.
After the first Gulf War, it was reported that crops in
Kuwait could be grown in soil that had been contaminated
with up to 10% crude oil and that over time and the presence
of these plants the concentrations of the PAH decreased
(Radwan et al. 1995).
G
<
unther et al. (1996) investigated the effect of ryegrass
(
Lolium perenne
L.) on the removal of hydrocarbons such as
PAHs from contaminated soils under laboratory conditions.
They reported that relative to contaminant decreases in
unplanted controls, the planted treatments produced faster
rates of contaminant disappearance, and increased microbial
abundance. Because less than 0.1% of the total contaminants
added were recovered from the plants themselves, it was
assumed that the losses were attributed to soil microbial
oxidation in the rhizosphere. A summary of the potential
fate of PAHs in plants is shown in Fig.
13.9
.
€
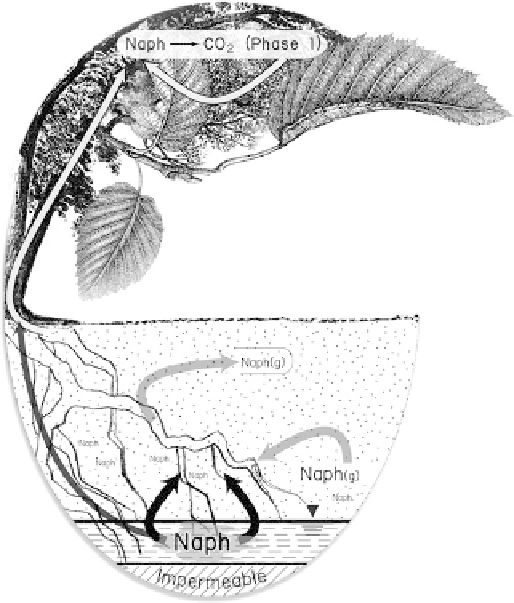
Fig. 13.9
Plant and groundwater interactions where PAH-
contaminated (naphthalene, as Naph, shown as an example) groundwa-
ter exists. Naph(g) indicates the volatile phase.
though catalytic converters were mandated standard on car
exhaust systems produced after the 1980s.
To improve combustion, additives that included lead-
substituted organic compounds were mixed into gasoline
stocks. Such leaded gasoline had high octane ratings, and
the lead was deposited during detonation on the value guides
and heads to protect the engine from excessive valve-train
wear. Because lead built up on the valves over time,
compounds were added to the gasoline to remove or scav-
enge these lead deposits. These lead scavengers included the
halogenated organics EDB and 1,2-dichloroethane (1,2-
DCA). The use of leaded gasoline was banned in the mid-
1970s.
In an attempt to decrease the air-quality degradation
resulting from the incomplete combustion of fuel in
automobiles, the U.S. Congress mandated that in pollution-
prone areas that fuel be supplemented with oxygenates, or
petroleum compounds to which an oxygen molecule has
been added, called reformulated gas (RFG). The oxygenates
typically used are ethers or alcohols, with the form R
13.4
Plant Interactions with Fuel Oxygenates
and Additives
The term “fuel” can represent any reduced, hydrogen-
saturated organic compound that when burned in the pres-
ence of oxygen releases its chemical bond energy, originally
supplied by plant photosynthesis, and converted to mechan-
ical energy. Essentially it is the reverse of photosynthesis.
Fuels can be a solid, such as wood or coal, a liquid, such as
gasoline, or a gas, such as methane, butane, or propane, or a
mixture, such as liquefied petroleum gas (LPG).
The consumption of liquid fuels, such as gasoline, is of
global scale and large volume. Considering that CO
2
and
H
2
O are supposed to be the only combustion byproducts, as
indicated by the products of the respiration reaction, it may
come as a surprise that this combustion has led to air-quality
degradation. This is because the organic source of the fuel
also contains nitrogen and sulfur compounds that, when
oxidized in an internal combustion engine, produces oxides
of nitrogen and sulfur, as NO
x
and SO
x
emissions, even
O
R
or R
OH, respectively, where R denotes an organic com-
pound. The RFG mandate called for a 2% by weight content
of oxygen. The compound MTBE was selected to meet this
oxygenate mandate. It could be sourced from waste stocks,
and it mixed easily with existing product flow in pipelines,


Search WWH ::

Custom Search