Environmental Engineering Reference
In-Depth Information
solubility's, a trait that also makes them more likely to be
placed on the USEPA's priority pollutant list.
Chemicals with a higher log
K
ow
and tend to be hydro-
phobic may enter the plant or be absorbed to the root tissues,
but these chemicals may become bound in plant tissues, and
lead either to bioaccumulation or to biotransformation, as
will be discussed later in this chapter. Briggs et al. (1982)
noted that this affinity for root uptake approached equilib-
rium rapidly, often being less than 48 h. Chemicals that have
a lower log
K
ow
are anticipated to have low uptake into
plants, because these compounds may be too soluble and
therefore not be able to pass through the root lipids. This
relation between the physical property of a contaminant and
its potential for uptake and fate in plants is a predictive
relation, not a deterministic one (Fig.
12.1
).
In a classic study, it was observed that the organic chem-
ical lindane was taken up by food crops such as carrots at
higher amounts if grown in sandy soils relative to more
organic-rich soils (Lichtenstein 1959). Essentially, uptake
of lindane was decreased in the organic soils because of
the increased partitioning of the organic lindane onto the
organic matter present in soils, which decreased the concen-
tration of lindane in the pore water available for uptake by
the plant. This example illuminates that in a general sense,
the difference in plant uptake is directly related to the physi-
cal and chemical properties of the contaminant, the physical
and chemical properties of the soil it is growing in, and the
chemical characteristics of the individual plant. The more a
chemical is absorbed by organic matter in the soil, or onto
roots in the soil, the less likely it will be taken up into the
xylem by plants.
As would be expected from the results of Lichtenstein
(1959), hydrophobic chemicals will be retained on soil
organic matter and not be taken up readily by plants. This
is especially true for those compounds whose log
K
ow
is
greater than 3.5. As a result, such contaminants actually
become part of the soil organic horizon and in this way
become sequestered from the more labile cycling and flow
of carbon. These contaminant carbon compounds also can be
bound to the cell membranes in the apoplast or the Casparian
strip of the endodermal tissue of plants. Hydrophilic
compounds with log
K
ow
<
1 have a different fate—these
compounds readily move through the plant apoplast, but not
through cell membranes, so transport across the endodermis
is limited. There are exceptions to this rule, such as is
evident by the presence of MTBE in the transpiration stream
of plants.
Some contaminants in groundwater sorb onto the immo-
bile organics present in aquifers and, therefore, these
contaminants are assumed to be less mobile relative to
contaminants that do not sorb onto organics. In some cases,
however, a portion of the organics present in aquifers are in
the dissolved and more mobile phase—contaminants sorbed
onto such dissolved organic matter (DOM) can be mobilized
in groundwater, and the process is referred to as facilitated
transport. The DOM consists of humic acids, which occur
naturally as a result of production by both living and dead
plants.
Groundwater contaminants, such as polycyclic aromatic
hydrocarbons (PAHs), also can become more bioavailable
(i.e., dissolved in water) for subsequent uptake by plants
(Wilcke 2000). A laboratory method developed by Tao
et al. (2006) utilizes accelerated solvent extraction with
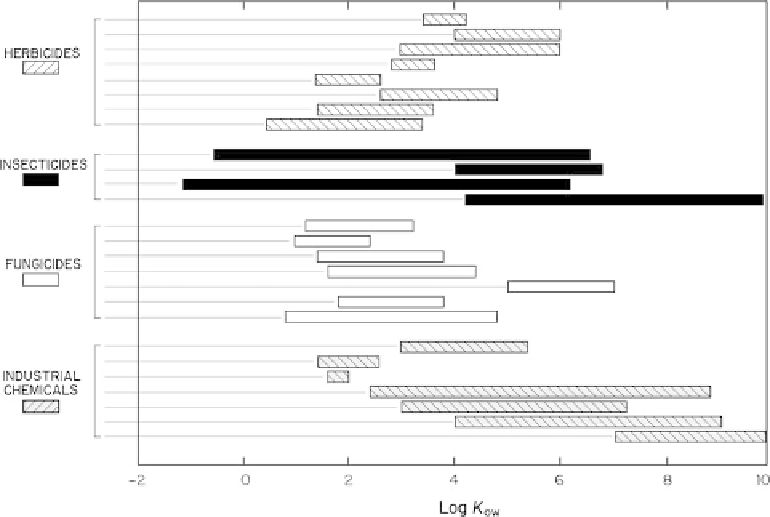
Fig. 12.1
Log
K
ow
for various
organic compound classes that
may be detected in groundwater.

Search WWH ::

Custom Search