Environmental Engineering Reference
In-Depth Information
during electron transport to an acceptor, such as oxygen
(Fig.
11.2
).
The rate of chemical reactions in these flows is important,
because common knowledge suggests, for example, that
sugar stored in the cupboard tends not to disappear, even
though ample supplies of oxygen are present. Sugar does not
spontaneously react with oxygen to form CO
2
and H
2
O
because for this reaction to proceed requires the input of
energy. This energy input, or activation energy, typically is
derived from catalysts or enzymes. The role of enzymes in
biochemical reactions was first investigated when yeast was
ground up with sand, which resulted in non-living matter
that was still able to cause the grape sugar to ferment
because the disrupted cells released enzymes (B
€
uchner
1897).
The interface between plants and groundwater brings to
light a few concepts with regard to the general carbon cycle
(Fig.
11.4
). A plant needs CO
2
,H
2
O, and light to make its
own food. It also needs the essential and trace nutrients to
synthesize its macromolecules, such as proteins and fats.
Terrestrial plants, therefore, depend on the atmospheric
part of the carbon cycle for CO
2
as a gas, and do not need
input of dissolved carbon, such as bicarbonate. Even aquatic
plants take in free, dissolved CO
2
in the water column for
fixation, and if dissolved CO
2
is limiting, will take up the
CO
2
in the form of bicarbonate ions, as HCO
3
. There is no
analogy for plants to uptake other forms of reduced carbon,
such as fuels, or oxidized forms of carbon, such as
chlorinated solvents, to support photosynthesis or growth
or macromolecule production.
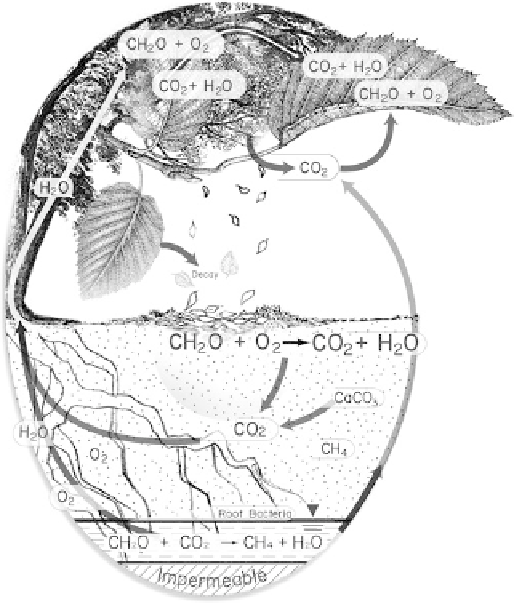
Fig. 11.4
A representation of the carbon cycle. Not only do plants take
up carbon dioxide but also release it during respiration. The net release
of carbon dioxide is an important metric when it comes to looking at
carbon dynamics in plants
.
Shown are water (H
2
O); oxygen (O
2
);
carbon dioxide (CO
2
); carbohydrate (CH
2
O); calcium carbonate
(CaCO
3
), and; methane (CH
4
).
terrestrial plants, oxygen enters plants as a gas through
the stomata and lenticels. Oxygen entry into plants is
by diffusion along a concentration gradient from higher
concentrations in the atmosphere (about 20%) to lower
concentrations (or partial pressures) in the subsurface.
Oxygen transport through a plant to roots is through
interconnected air-filled spaces that exist between loosely
packed cells collectively called the cortex. Oxygen is con-
sumed during respiration, and oxygen also can diffuse into
the rhizosphere, at a rate controlled by the oxygen concen-
tration gradient present and potential abiotic and biotic
sinks for oxygen.
The electron acceptors in the TCA cycle are as integral
as the electron donors. The compound NAD (nicotinamide
dinucleotide) is the electron acceptor for three of the four
oxidation steps. The oxidized version of the compound,
NAD+, (NAD is written as NAD
+
, much like NH
4
+
to
NH
3
) is reduced by two electrons to form NADH.
Dinucleotides are simply two mononucleotides linked by
the phosphate groups. Electrons are passed between the
reduced organic carbon and NAD to form NADH, much as
a baton is passed between relay runners; NADH has reduc-
ing power. Energy is stored by ATP synthesis and released
11.1.4 The Flow of Nitrogen
Similar to animals, including man, plants need an external
source of nitrogen in order to make proteins, such as amino
acids, NH
2
, or enzymes. With respect to the total dry weight
of most plants, compounds that contain nitrogen approach
1-3%, and more than half of this total amount is stored
in the leaves. Nitrogen also is used in the manufacture of
non-protein compounds, such as hormones, and defensive
chemicals, such as alkaloids, which are discussed in the next
section of this chapter.
Unlike the direct uptake of CO
2
that exists in the atmo-
sphere at low, part per million concentrations, nitrogen in the
atmosphere is unavailable for use by plants even though it is
present at levels near 80%, or is not bioavailable in the form
of insoluble organic matter in soils or rocks. Most of the
nitrogen in soil is in the form of organic matter, such as leaf
litter and, therefore, available to plants only after microbial
and fungal decay and release.
For nitrogen to become bioavailable to plants, certain
processes must occur. For example, plants have adapted

Search WWH ::

Custom Search