Environmental Engineering Reference
In-Depth Information
as iron, often seen as the iron-oxide rich deposits in many
near-surface geologic deposits.
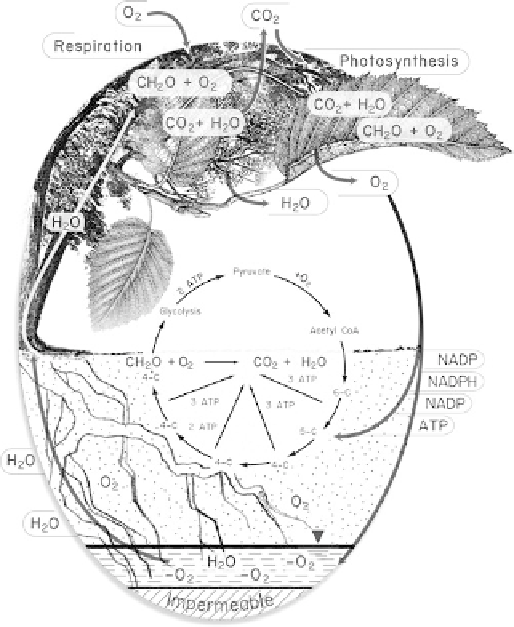
Although oxygen is a waste product of photosynthesis,
plants are aerobic creatures that also require oxygen to
release the energy stored in the food produced by photosyn-
thesis for growth and metabolism (Fig.
11.2
). Plant tissues
above ground where oxygen usually is not limited and below
ground where oxygen can be limited require oxygen to
produce ATP. Oxygen is limited in some soils, the vadose
zone, the capillary fringe, and groundwater because it is
consumed rapidly by both biotic and abiotic reactions and
has low solubility in water and low diffusivity in water
relative to air. If an ample amount of oxygen is present,
glucose formed during photosynthesis can be oxidized to 6
units of CO
2
and H
2
O and produce 38 units of ATP. This is
much higher than the production of two units of CO
2
and
ethanol and only two units of ATP under anoxic conditions
where fermentation occurs.
The production of oxygen by photosynthesis and its con-
sumption by aerobic metabolism is near a steady-state condi-
tion such that the excess in the atmosphere approaches 20%
oxygen by volume. There are areas of the planet, however,
where oxygen consumption exceeds oxygen production. In
many surface-water systems, for example, photosynthetic
organisms release excess oxygen to the water column on a
daily basis, but at night this dissolved oxygen (DO) is
depleted by aerobic respiration. In the sediments of wetlands
and some aquifers a few inches to several feet below the water
table, DO concentrations also are low because the rate of
oxygen consumption is higher than input by recharge or gas
exchange with the air in the unsaturated zone. Moreover,
these oxygen dynamics are exacerbated by the low solubility
of oxygen in water, no greater than about 9 mg/L at room
temperature and atmospheric pressure.
It took centuries of scientific discovery to link the two
processes of photosynthesis and aerobic respiration together.
Until the 1700s, air was thought to be one element only, not
a mixture of elements as we recognize today. Work by
Stephan Hales (1677-1761), Joseph Black (1728-1799),
Karl W. Scheele (1742-1786), Joseph Priestley
(1733-1804), and John Mayow (1640-1679) and later by
the chemist A. Lavoisier (1743-1794) began to show that air
consisted of many different parts, and that one part was
oxygen. The candle-and-mouse experiments performed ear-
lier by Robert Boyle (1627-1691) had indicated that
removal of air from a sealed jar resulted in extinguishing
the candle and the life of the mouse, but the question
remained whether or not one or more elements in the air
was responsible. This question was solved by John Mayow
when introduction of both candle and animal at the same
time resulted in earlier cessation of flame and life than when
they were added at different times.
Other observations of the burning candle used during this
experiment indicated that as the flame was allowed to con-
tinue, the wax was used up. This led most scientists to the idea
that oxygen in the air supported the flame, but the loss of mass
of the candle to the air during burning was responsible for the
loss of wax. The context of the time in the mid-1700s was
called the “phlogiston theory” that stated when materials were
burned a substance called phlogiston, from the Greek, mean-
ing inflammable, was released. The heat and flame left the
item undergoing combustion, or being detached from the
ashes left behind after a wood fire, for example.
The experiments of Lavoisier refuted the presence of
phlogiston by his use of quantitative approaches. He argued
that the material undergoing combustion was supported by
the removal of oxygen from the air. In fact, he was able to
demonstrate that some materials undergoing combustion
gained
rather than
lost
weight. He was able to make this
statement because he methodically collected measurements
of weights during his experiments, and thusly contributed in
Fig. 11.2
A representation of the flows of oxygen and carbon during
plant and groundwater interaction. Plants, like mammals, are aerobic
life forms and respire, so plants consume as well as release oxygen.
Roots can live in saturated soils or groundwater as long as oxygen is
present, either from recharge or diffusion through the plant physiologi-
cal structure or soil-pore spaces. Shown are water (H
2
0); oxygen
(O
2
,
O
2
is anoxic); carbon dioxide (CO
2
); carbohydrate (CH
2
O);
adenosine triphosphate (ATP); nicotinamide adenine dinucleotide
phosphate (NADP); nictotinamide adenine dinucleotide phosphate,
reduced (NADPH) (Glycolysis is discussed in the section on carbon
flow).

Search WWH ::

Custom Search