Environmental Engineering Reference
In-Depth Information
metabolism. An example of a plant that fixes carbon this way
is the epiphyte Spanish moss. The pathway is the same as for
the C
4
plants, with a pre-fixation step, but differs in that the
C
4
plants have the two steps separated by
location
, and the
CAM plants have the two fixation steps separated by
time
.
For example, the final fixation of carbon in CAM plants
occurs in the dark of night by the same PEP enzyme,
which produces malic acid which is then stored in cell
vacuoles. Upon the return of light the next day, the malic
acid is then decarboxylated to CO
2
, which can then undergo
fixation by the rubisco enzyme. This difference in biochem-
ical reactions between C
4
and CAM plants produces differ-
ent oxygen and hydrogen isotope ratios in plant water
(Sternberg et al. 1986).
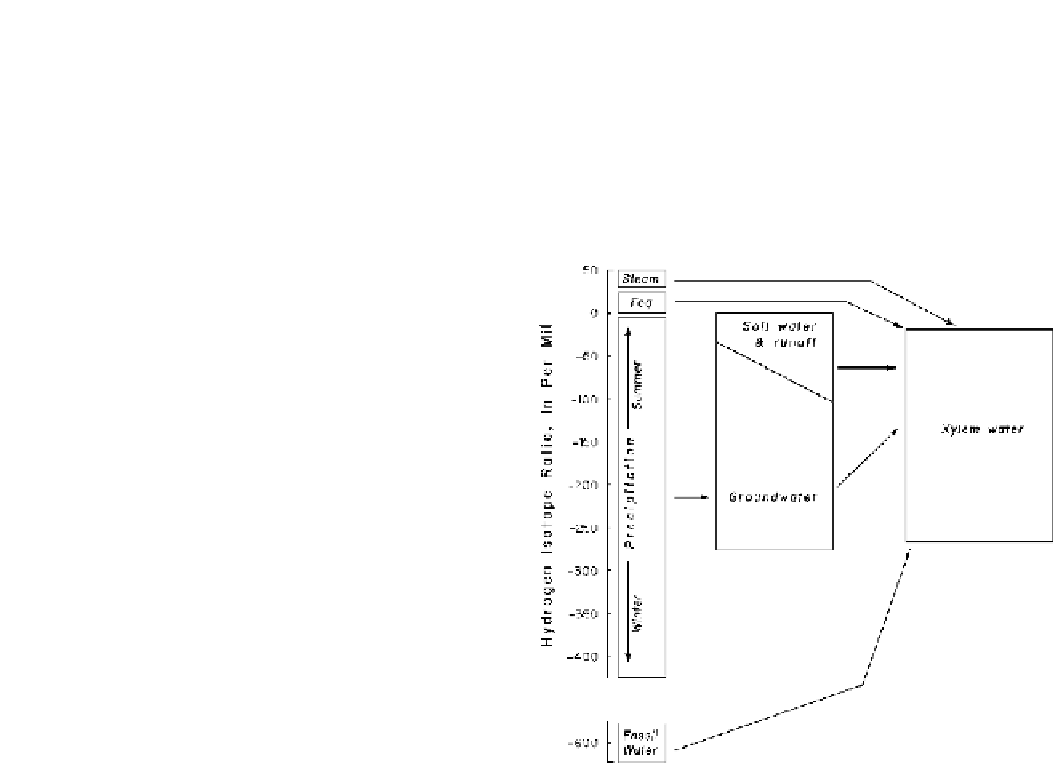
In addition to using the stable isotopes of carbon, the
stable isotopes of water also can be used. The stable isotopes
of water, as deuterium, as
various sources of water and, therefore, water in plant tran-
spiration is depicted in Fig.
9.12
.
One of the first studies that proved the usefulness of stable
isotopes in elucidating the source of water used by trees was
performed by White et al. (1985) (Fig.
9.13
). In a swamp in
Arkansas, the stable H isotope composition of precipitation
was greater than
12
per mil. The sap of baldcypress (
Taxodium distichum
) trees
30 per mil and the groundwater was
18
O, in tree
tissues can be compared to values of potential sources of
water (White et al. 1985; Dawson 1993; Scrimgeour 1995).
All the potential sources of water for plants should be sam-
pled and analyzed for these isotopes, including precipitation,
soil moisture, runoff, surface water, fog, dew, and ground-
water. Although all these sources are derived from precipi-
tation, the various processes that affect the water in these
components affect the stable isotope value and, therefore, for
a specific area often will have diagnostically unique values.
For example, evaporation of water will occur to surface
waters to a greater extent than to groundwater, even if
precipitation of the same stable isotopic composition is
the source, and this evaporation affects the stable isotope
composition of the water and water vapor, due to mass
differences between H and D, as well as vapor pressures.
Because of the wide variation in the stable isotopic compo-
sition of precipitation around the globe, most results will be
site specific and cannot be readily comparable to other site
results.
The reason that the stable isotope ratio of the various
water sources can be used is that there is no fractionation
of isotopes when water is taken up by those plants that
cannot exclude salt, so that the stable isotope ratio of the
source water(s) is preserved. The signatures are preserved
because the entry of water into the root hairs is by mass
diffusion, rather than molecular flow, so the water is not
subject to kinetic fractionation. However, due to the trans-
formation of liquid water to vapor in the leaves, the stable
isotopic composition
will
be affected in these tissues, as well
as in stems that are not completely suberized and, therefore,
exchange gas with the atmosphere. The water in leaves will
be isotopically enriched, or contain a greater percentage of
the heavy isotope on account of discrimination during water
vapor evaporation from the open stomata (Farquhar et al.
2007). Samples also can easily be obtained and routinely
analyzed. The range of values that can be expected of
2
H, and oxygen, as
d
d
Fig. 9.12
Stable hydrogen isotopes of various water sources available
to plants (Modified from Dawson 1993). Xylem is shown for woody
plants.
Fig. 9.13
Stable hydrogen isotopes (as
d
D, or deuterium) of precipi-
tation, groundwater, and plant water (Modified fromWhite et al. 1985).

Search WWH ::

Custom Search