Biomedical Engineering Reference
In-Depth Information
substitution of phosphorothioate bonds for the phosphodiester
bonds and 2
′
sugar modifications (e.g., a fluorine substitution) [21,
47, 148]. However, the selective incorporation of 2
′
O-methyl groups
on the sugar backbone has been the most common substitution used
for the protection of siRNAs from serum nucleases and the reduction
of off -target eff ects, including the inhibition of the proinflammatory
eff ects of some siRNAs and the targeting of partially complementary
mRNAs. The incorporation of 2
′
O
-methyl modifications within
the seed region of the guide strand of the siRNA reduced both the
number and the magnitude of off -target silencing without adversely
aff ecting the on-target silencing efficacy [74].
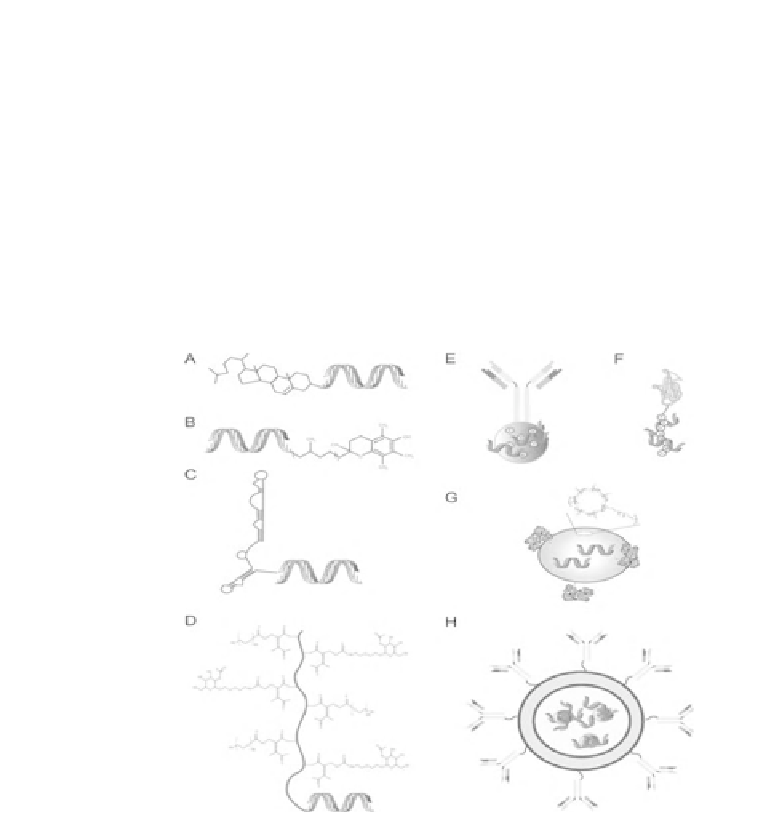
Figure 6.3
Strategies for cell type-specific siRNA delivery
in vivo
. A
variety of approach have been used for the cell type-specific delivery
of siRNAs, including the direct conjugation of targeting molecules,
such as, ligands (cholesterol (A) and a-tocopherol (B)), aptamers (C),
or polymers (Dynamic polyconjugates (D). In addition, the negatively
charged siRNA will spontaneous bind to positively charged proteins
or peptides fused to an antibody (E) or a ligand (eg., a portion of the
rabies virus glycoprotein (RVG)). SiRNAs can be encapsulated into
polymeric (eg. cyclodextrin (G) coated with the ligand transferring)
or lipid nanoparticles (eg., immunoliposomes (H), lipid nanoparticles
coated with an antibody).














Search WWH ::

Custom Search