Biomedical Engineering Reference
In-Depth Information
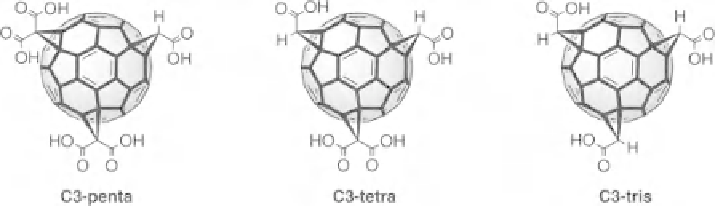
Figure 19.3
Schematic representation of the C3 decarboxylation products C3-penta, C-tetra, and
C-tris. Only one stereoisomer each is represented although they were formed as mixtures of isomers
(NMR).
20
C.
In solution, the stability of C3 (1) is highly variable, depending upon the suspending
medium. In DMSO at room temperature, C3 (1) is degraded completelywithin a few
minutes; the same is true in DMSOwith immediate freezing at
degrades approximately 0.5%per week at room temperature, but is stable at
20
C. C3 solutions
of distilled water, phosphate-buffered Tyrode's solution, and 5% glucose exhibit a
degradation rate of approximately 4% per week at room temperature, but all three
solutions are completely stable at
20
C for at least 10 weeks. C3 solutions in
polyethylene glycol (PEG) at room temperature exhibit rapid degradation rates of
approximately 40% per week.
HPLC analysis of C3 degradation products indicates that decarboxylation
reactions of the malonyl adducts represent the major pathway for degradation of
C3 (1). Three major decomposition products, namely, the mono-, bis-, and tris-
decarboxylation products C3-penta, C3-tetra, and C3-tris, could be identified
(Fig. 19.3). The initial breakdown product is mostly C3-penta, but with continued
degradation, significant amounts of C3-tetra and C3-tris are observed as well. In the
presence of DMSO, complete degradation to C3-tris appears within 1-2 min at room
temperature.
19.3.3 Toxicity of Water-Soluble Fullerenes
in Zebrafish Embryos
We compared the overall toxicity of our water-soluble fullerenes in zebrafish
embryos, and the results are summarized in Table 19.2. Fullerenes were added to
the water in single wells of a 96-well plate, each containing a single zebrafish embryo
at 24hpf, and lethality alongwith any observedmorphologic abnormalities was scored
at 120hpf. In most cases, fullerenes were tested at varying concentrations up to
500
M due to
solubility issues at higher concentrations. LC
50
was calculated as the fullerene
concentration at which 50% lethality is observed at 120hpf. For the three cationic
fullerenes 10-12 tested, the LC
50
was less than 120
m
M, but in some cases the maximum concentration tested was 250
m
m
M, and in the case of 12,aslowas
30
m
M. For the anionic fullerenes, the LC
50
values were considerably higher reaching
Search WWH ::

Custom Search