Biomedical Engineering Reference
In-Depth Information
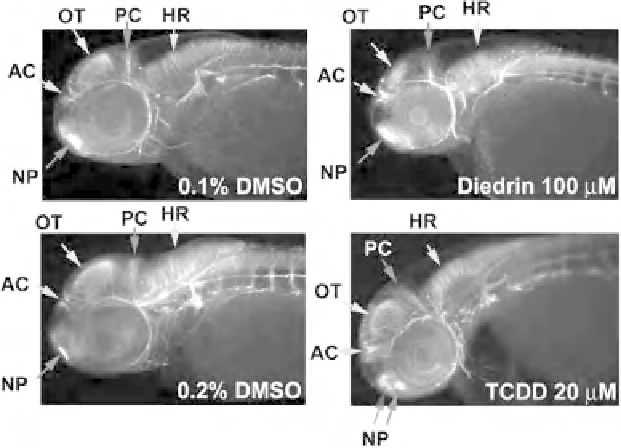
Figure 10.8
Visual assessment of axon tracts in the brain of control and compound-exposed embryos
at 48hpf. AC, anterior commissure; HR, hindbrain region; NP, nasal placode; OT, optic tectum; PC, post
erior commissure. Anterior, left and dorsal, up.
across all neuroanatomical end points (Ton et al., 2006). Overall, these results showed
strong correlation with mammalian data and suggest that zebrafish is a predictive
animal model for developmental neurotoxicity screening.
10.4 SUMMARY
Zebrafish have been shown to be a predictive animal model for assessing neurotox-
icity. The ability to visually examine several distinct end points and to elucidate the
mechanisms of toxicity
in vivo
is a significant advantage of zebrafish as a model for
assessing neurotoxicity morphologically. Many qualitative and quantitative toxicity
end points can be rapidly examined in the intact zebrafish nervous systemwithout the
artifacts that can result from dissecting organs. Genetic tools, including mutants and
transgenic and gene knockdown animals, can be used to assist in developing
comprehensive morphological and behavioral studies. Technology and instrumen-
tation including microplate readers, digital image systems, and fluorescence-
activated cell sorting have been adapted for quantitative analysis of drug effects in
zebrafish, significantly increasing assay reliability and efficiency. Quantitative studies
including 3D computer analysis for detecting gene expression, automated image
analysis for quantitative neuronal phenotyping, and morphometric analysis for
quantifying hair cells have been reported (Ton and Parng, 2005). Although many
qualitative end points for predicting neurotoxicity in mammals can be assessed in
zebrafish, direct comparison between results in zebrafish and results in mammals
Search WWH ::

Custom Search