Biomedical Engineering Reference
In-Depth Information
Scheme 3.9
Synthesis of 2-Se-uridine phosphoramidite and RNAs. Reagents and conditions: (
a
)
2-Thiouracil, TMS-Cl, HMDS, reflux; (
b
)SnCl
4
,C
2
H
4
Cl
2
,
20
ı
C; (
c
)NaOCH
3
, MeOH; (
d
)
DMTr-Cl, pyridine, rt; (
e
)CH
3
I, DBU, DMF; (
f
)Se,NaBH
4
, EtOH; (
g
) TBDMS-Cl, imida-
zole, DMF; (
h
)ICH
2
CH
2
CN, (i-Pr)
2
NEt, CH
2
Cl
2
;(
i
)(i-Pr
2
N)
2
P(Cl)OCH
2
CH
2
CN, (i-Pr)
2
NEt,
CH
2
Cl
2
;(
j
) solid-phase synthesis
pair [
17
]. X-ray crystallographic results have revealed that the bulky selenium
atom can be accommodated to the minor groove in
2-Se
U/A pair (Fig.
3.7
), while
the H-bond lengths between N3-U and N1-A as well as O4-U and N6-A are
significantly shortened. These changes are probably the main reasons of the 2-Se-U
discrimination against G, in addition to the steric and electronic effects of the
2-exo-selenium atom disrupting the wobble H-bond formation. The Se-electron
delocalization, which increases the base-stacking interaction, may also explain the
higher stability of the 2-Se-U-modified RNA duplexes than the corresponding native
ones [
17
].
3.4.2.4
6-Seleno-Deoxyguanosine
The site-specific selenium modification of purine nucleobase in DNA was also
accomplished in 2008 [
86
]. Similar to the 4-Se-T synthesis, 6-Se-dG was made
by selective activation of carbonyl group on the 6-position of guanine with
TIPS-Cl (2, 4, 6-triisopropylbenzene-1-sulfonylchloride); then the activating
group was displaced by cyanoethyl selenide. The resulting 6-Se-dG nucleoside



















































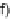









































































































































































Search WWH ::

Custom Search