Biomedical Engineering Reference
In-Depth Information
Scheme 3.8
Synthesis of 2-Se-T phosphoramidite and DNAs. Reagents and conditions: (
a
)
DMTr-Cl, pyridine, DMAP, rt; (
b
)DBU,DMF,CH
3
I; (
c
)Se,NaBH
4
, EtOH; (
d
) I-CH
2
CH
2
CN,
i
-Pr
2
NEt, CH
2
Cl
2
;(
e
)(
i
-Pr
2
N)
2
P(Cl)OCH
2
CH
2
CN, (
i
-Pr)
2
NEt, CH
2
Cl
2
;(
f
) Solid-phase synthesis
was investigated. Though the 2-Se-derivatized nucleoside was synthesized over
three decades ago [
80
,
81
], 2-Se-T was never incorporated into DNA due to the
synthetic difficulties in the Se functionality protection and deprotection. Recently,
we have developed a novel synthetic route (Scheme
3.8
) to incorporate the selenium
functionality by displacing the 2-Se moiety with the selenium functionality [
18
],
which is a convenient and efficient strategy for the selenium incorporation. The key
selenization reaction is accomplished by displacing the 2-methylsulfide activating
group with sodium hydrogen selenide. This reaction is also called the Huang
reaction. Later, the 2-Se-T phosphoramidite was successfully synthesized and
incorporated into DNA for studying the base-pair recognition and duplex structure.
This novel synthesis of 2-Se-T utilizes 2-methylthio-T as an active intermediate,
followed by the methylation and the selenizing reaction (Huang reaction) with
NaSeH to give 2-seleno-T (Scheme
3.8
). To ensure the stability of the selenium
functionality during solid-phase synthesis, cyanoethyl group is used to protect it,
preventing the deselenization in solid-phase synthesis cycle. Biophysical experi-
ments of the 2-Se-T-DNAs have demonstrated largely increased base-pair specificity
by destabilizing T/G (wobble pair) and T/C pairs. Although the thymidine 2-exo-
position is not involved in Watson-Crick base pairing (or hydrogen bonding), it
is involved in the wobble T/G pairing. The 2-Se moiety discriminates against the
wobble pairing via the steric and electronic effects of the selenium atom. The
crystallographic characterization also suggests that 2-Se-T has higher base-pairing
specificity over native T (Fig.
3.6
). This result was also later supported by the
computational study [
82
].










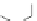




























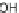






















































































































Search WWH ::

Custom Search