Biomedical Engineering Reference
In-Depth Information
Scheme 3.6
-Se-phosphate modifi-
cation. (
a
) 2-Chloro-4H-[1, 3, 2]-benzodioxaphosphorin-4-one (salicyl phosphorochloridite) and
pyrophosphate. (
b
) Benzo-1,2-thiaselenol-3-one (BTSE) and Et
3
N, dioxane. (
c
) Diluted NH
3
Synthesis of deoxy- and ribonucleoside triphosphates with
'
DNA and PSe-RNA synthesis. The conditions of the enzymatic reactions are
mild, and the synthesized PSe-nucleic acids are diastereoisomerically pure, which
allows us to make longer oligonucleotides with the PSe groups incorporated at
various sites. We have developed the synthesis (Scheme
3.6
)ofSe-dNTPsandSe-
NTPs (
-phosphoroselenoate triphosphates) [
28
] and the Se-nucleobase-modified
triphosphates [
58
]. Moreover, we have found that they can be generally recognized
by DNA as well as RNA polymerases [
88
-
90
]. More interestingly, after the
modification of a catalytic RNA (hammerhead ribozymes) by PSe, it can retain its
activity up to the native level.
'
3.4
Nucleobase Modifications of Nucleic Acids
with Chalcogens
Simple replacement of the oxygen atoms on nucleobases with other chalcogen
elements (such as S and Se) can lead to numerous interesting properties. For
example, 2-thiothymidine could be applied to a synthetic biology system to
improve replication accuracy. Benner's lab has reported that the fidelity of PCR
reaction involving artificial base pairs (isoC/isoG) could be significantly improved,
simply by replacing TTP with 2-thioTTP in PCR reaction, since 2-thioT can
discriminate against isoG [
79
]. Enhanced base pairing and replication efficiency
of thiothymidines were also reported by Kool's lab [
59
]. The large chalcogen atoms
on nucleobases could be used to electronically and sterically alter the fidelity of
the base pairing. This interesting characteristic may prove useful in nucleic acid





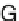














































































































































Search WWH ::

Custom Search