Biomedical Engineering Reference
In-Depth Information
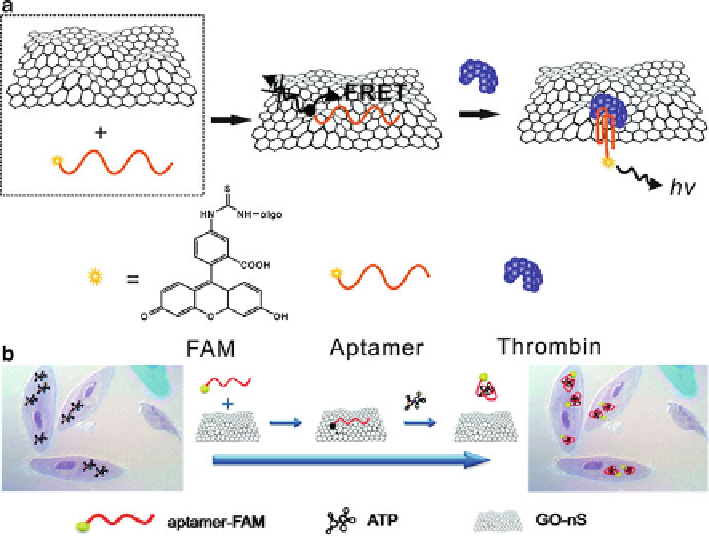
Fig. 13.11
(
a
) Schematic illustration of GO-based aptamer sensor for thrombin detection
(Reprinted with the permission from Ref. [
177
]. Copyright 2010 American Chemical Society).
(
b
) Schematic illustration of in situ ATP sensing in living cells by using aptamer/GO nanosheets.
(Reprinted with the permission from ref. [
179
]. Copyright 2010 American Chemical Society)
aptamer/GO nanosheet (GO-nS) for intracellular aptamer delivery and ATP sensing
in living cells (Fig.
13.11
b) [
179
]. Based on their design, aptamer-FAM/GO-nS
entered into JB6 cells (mouse epithelial cells) and then formed a target-aptamer
complex with cellular ATP, followed by fluorescence recovery. As a control, the
use of random DNA-FAM/GO-nS did not result in an obvious change in cellular
fluorescence. In addition to aptamers, Fan et al. [
180
]andZhangetal.[
181
]
independently reported GO-DNAzyme-based biosensors for fluorescence detection
of Pb
2C
.
Another promising sensing application of graphene is to incorporate graphene
into field-effect transistors (FETs) for the detection of a variety of biomolecules
[
182
,
183
]. As a microscale or even nanoscale device, graphene FETs (G-FETs)
exploit the changes in conductance when the molecules of interest adsorb on its sur-
face. Ohno et al. reported an aptamer-modified G-FET as a label-free immunosensor
for detection of immunoglobulin E (IgE), which is an antibody subclass found
only in mammals (Fig.
13.12
)[
184
]. To utilize the high carrier mobility in the
graphene channel, single-layer graphene was used for the modification with IgE
aptamers. An IgE concentration-dependent drop in the conductance was observed
Search WWH ::

Custom Search