Biomedical Engineering Reference
In-Depth Information
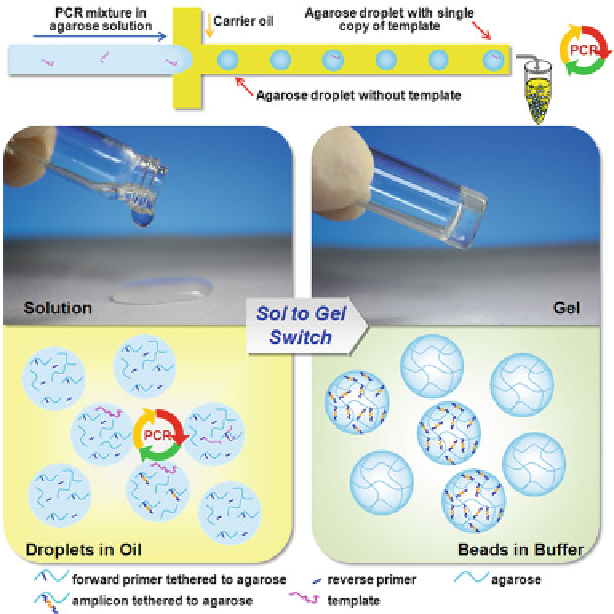
Fig. 7.4
Agarose-in-oil droplets for PCR amplification. Each agarose droplet contains free reverse
primer, while forward primer is conjugated to agarose. Droplets with DNA template will produce
amplicons physically attached to the agarose matrix after PCR. Following emulsion PCR, the
droplets are cooled to gelate to agarose beads for downstream analysis (Reproduced from Ref. [
23
]
with permission of The Royal Society of Chemistry, and the figure file is a gift from Dr. Chaoyong
James Yang)
cooling the solution below gelling point of agarose. Since PCR forward primer
is conjugated to agarose, amplicons can physically attach to the agarose matrix
after PCR. DNA products amplified in the droplet can retain their monoclonality
even after the oil phase is removed and afford flexible downstream processing
and analysis. A distinct advantage of this approach is the higher PCR efficiency
than solid beads encapsulation-based approach where PCR efficiency suffers with
its reaction carried out at the solid-liquid interface [
24
,
25
]. They also showed
the utility toward single template amplification, suggesting that this method can
be used for digital PCR applications (that can be used to absolutely quantify
or clonally amplify nucleic acids, see details in Sect.
7.3.1
). By adding another
aqueous channel that contains cell lysis buffer, the same group showed the utility of
single-cell lysis and following amplification using this platform [
26
]. As a versatile
platform, they employed this platform to screen aptamers [
27
]. The fluorescent gel
Search WWH ::

Custom Search