Biomedical Engineering Reference
In-Depth Information
(a)
(b)
(c)
Figure 5.1
Possible nanostructures present within microemulsions: (a) water-in-oil, (b) bicontinuous and
(c) oil-in-water (adapted from Garti
et al
., 2005).
The preparation of microemulsions requires the delicate admixture of an aqueous phase,
an oil phase and a surfactant phase. Often, co-surfactants are added to aid in formation and
to extend the range of compositions leading to microemulsion formation for a given set of
ingredients. Figure 5.1 shows the three known types of microemulsions: (a) water-in-oil
(w/o) (L
2
phase), (b) bicontinuous (comparable amounts of oil and water) and (c) oil-in-
water (o/w) (L
1
phase). As shown in Figure 5.1, a duplex interfacial film separates the oil
and aqueous phases. The formation, properties and stability of a microemulsion strongly
depend on the number of extent of interactions between the functional groups present in the
components solubilized in the oil, aqueous and interfacial regions. Other structures, such as
liquid crystals and gels, may also exist, either alone or in combination with true microemul-
sion phases (Hamdan
et al
., 1995 ; von Corswant and Soderman, 1998 ; Kunieda
et al
., 1999 ;
Garti, 2003 ; Yaghmur
et al
., 2005 ).
Though they are seeing increasing use in both food and other applications, notable
limitations to microemulsion usage exist, particularly for food-compliant applications. These
include:
(1) difficulty in solubilizing long-chain triglycerides due to their relative lack of molecular
flexibility;
(2) the limited choice of food-grade surfactants and co-surfactants due to toxicity concerns;
(3) the amount of surfactant required for microemulsion formation is often higher than
permitted by legislation;
(4) non-dilutable microemulsions have limited usage in food products;
(5) elimination and substitution of alcohols with other acceptable food-grade surfactants/
co-surfactants whilst maintaining microemulsion integrity can be challenging, although
progress has been made in developing alcohol-free microemulsions (Constantinides and
Scalart, 1997 ; Polizelli
et al
., 2006 ).
5.2 WINSOR CLASSIFICATION/PHASE BEHAVIOR
Winsor (1948) developed an approach to classify equilibrium systems consisting of
mixed water, oil and surfactants (Figure 5.2), which is still used to this day. The four
categories are:
(1) Type I: o/w microemulsion in equilibrium with excess oil.
(2) Type II: w/o microemulsion in equilibrium with excess water.
(3) Type III: bicontinuous structure in equilibrium with excess oil and water.
(4) Type IV: single phase microemulsion (o/w or w/o) with no excess oil or water.









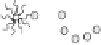















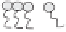

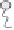


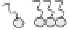




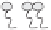





















Search WWH ::

Custom Search