Biomedical Engineering Reference
In-Depth Information
Reverse micelle
(containing protein)
Organic phase
Empty reverse
micelle
Protein of
interest
Other components in
solution
Aqueous phase
Figure 3.5
Schematic representation of the extraction of proteins using reverse micellization.
Protein solubilized in the reverse micellar solution can be transferred back into an
aqueous solution by contacting the micellar solution with an aqueous solution containing a
high concentration of salt (e.g., KCl, CaCl
2
), which has the capability to exchange with
protein in the micelles (Asenjo and Chaudhuri, 1996). Reverse micellization has been
successfully used to separate a variety of proteins including enzymes (e.g., lysozyme,
trypsin and ribonucease).
3.3.2.3
Protein recovery
Isoelectric precipitation
Most proteins precipitate at pH values close to their isoelectric point. This property can be
used to selectively precipitate different proteins from solution. As most food proteins have
their isoelectric point in the range pH 4-5, this pH range is frequently used for protein
recovery in the food processing industry. Typically, after alkaline, acid or salt extraction, the
pH of the protein extract is adjusted to the desired isoelectric point to induce protein
precipitation, followed by centrifugation to recover the protein, washing to remove salts,
neutralization and drying.
Salting out
Water from the hydrated protein is removed at high enough neutral salt concentrations (>1 M),
leaving the exposed proteins to interact with each other through hydrophobic interactions.
This results in protein aggregation and precipitation, a phenomenon known as “salting out”.
The Hofmeister series promote salting out, aggregation and stabilization or unfolding, dis-
sociation and salting in depending on the ascending or descending order of the ions:
SO
4
2-
< F
-
< CH
3
COO
-
< Cl
-
< Br
-
< NO
3
-
< I
-
< ClO
4
-
< SCN
-
, NH
4
+
< K
+
< Na
+
< Li
+
< Mg
+
< Ca
2
+
(Cheftel
et al
., 1985). Ammonium sulfate is frequently used for the selective precipitation of
proteins because it is relatively inexpensive and has high solubility. Other salts used in protein
applications are magnesium chloride (MgCl
2
) and calcium chloride (CaCl
2
).











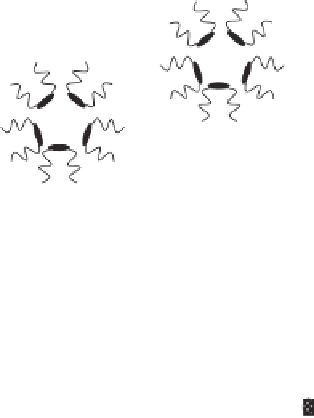






Search WWH ::

Custom Search