Biomedical Engineering Reference
In-Depth Information
2
H
3
C
SO
2
Cl
+
HO
6
CH
2
OH
CD
HO
3
p-TsCI
OH
2
O
H
3
C
SO
2
6
CH
2
OH
+
HO
3
OH
NH
2
p-TsCI
Chitosan
CD-2-TsCI
OH
O
OH
2
NH
6
CH
2
OH
HO
3
CD-2-chitosan
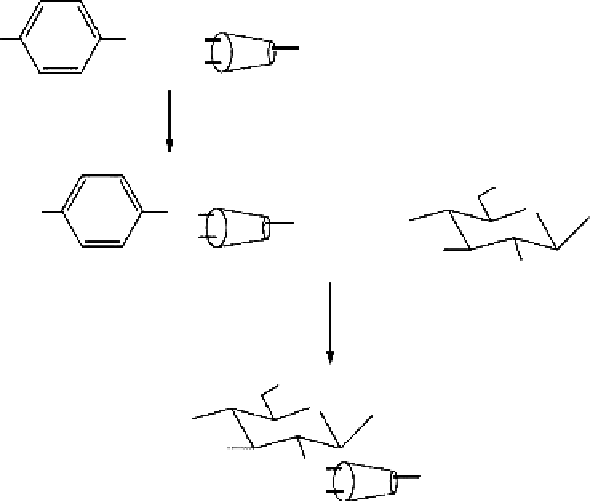
Figure 2.27
Reaction scheme for the synthesis of β-CD-graft-chitosan. (From Chen, S. and Wang, Y. 2001.
J Appl Polym Sci
82:
2414-2421. With permission.)
urethane product (-NH-COO-) due to the transfer of a proton from the hydroxyl group to
the nitrogen atom of isocyanate. In addition, isocyanate reacts with the hydroxyl groups of
β-CD to form a product similar to urethane [221]. It is assumed that the cross-linking of the
hydroxyl groups of chitosan with HMDI resulted in a chitosan-HMDI complex, which then
binds with the hydroxyl groups of β-CD to form β-CD-graft-chitosan. HMDI cannot bind
to the amino groups of chitosan due to the lower affinity for amino groups as compared to
hydroxyl groups under low pH value [221]. For these reasons, Sreenivasan [222] reported an
adsorbent matrix synthesized by coupling β-CD to chitosan using HMDI. The matrix
obtained in this study was found to be insoluble in organic as well as acidic or alkali media.
The extent of cholesterol removal by this matrix from the solution was studied. The results
indicated that nearly 21% of cholesterol was removed from the solution.
2.10.5 grafting of CD onto Chitosan by reductive Amination
Reductive amination is one of the major reactions applicable to the modification of chito-
san. Introduction of CD residue into chitosan has been successfully attained in a homoge-
neous system through a reductive amination strategy. CD derivatives with aldehyde
functional groups are useful to graft CD into chitosan by the formation of Schiff's base.
Tanida et al. [223] reported the synthesis of β-CD-grafted chitosan by the formation of
Schiff's base between 2-
O
-formylmethyl-β-cyclodextrin and chitosan in acetate buffer at
pH 4.4, followed by reduction with NaBH
3
CN. The product, which had a DS of 37%, was
found to be soluble in water at neutral and alkaline conditions. 2-
O
-formylmethyl-α-CD
Search WWH ::

Custom Search