Biomedical Engineering Reference
In-Depth Information
(a)
Tr
N
N
Tr
O
O
OH
O
A
B
O
O
O
O
O
HO
HO
HO
NH
NH
NH
O
O
O
n
n
n
Cl
+
-
N
+
-
N
l
Cl
N
N
(b)
Tr
OH
O
B
O
-
O
l
O
HO
OH
O
NH
HO
N
+
N
NH
O
n
O
O
n
C
Tr
N
O
N
+
-
O
N
Cl
+
O
HO
-
N
l
NH
2
n
C
-
-
Tr
l
OH
l
+
O
OH
O
N
+
N
B
O
O
HO
O
O
HO
NH
NH
O
n
O
n
+
-
+
Cl
N
-
l
N
+
+
-
N
Cl
-
N
l
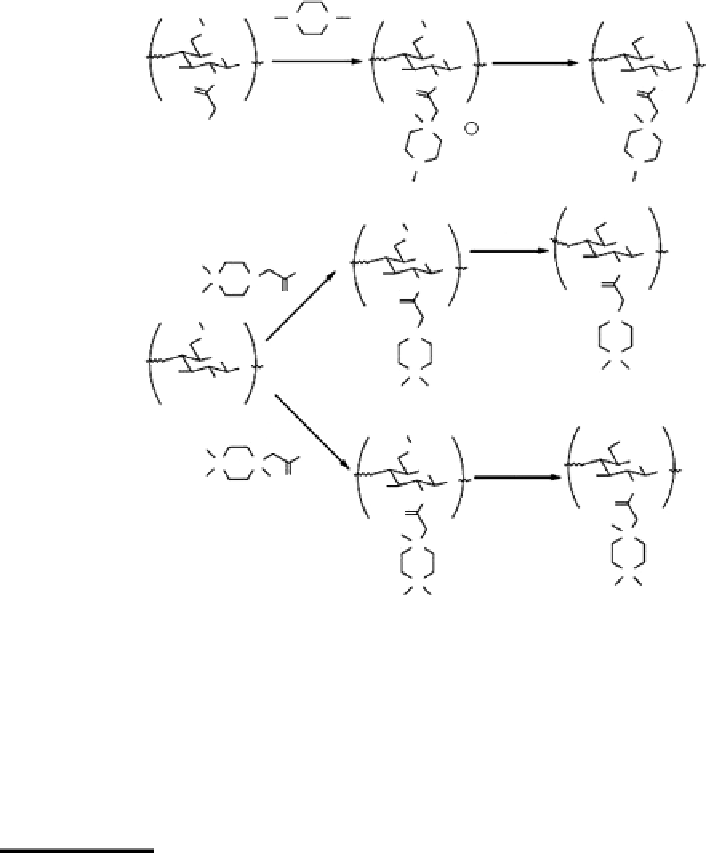
Figure 2.10
Synthetic route for the preparation of quaternary piperazine derivatives of chitosan: (A) KI, NMP, 60°C, (B) aq.
HCl, room temperature, and (C) DCC, HOBt, NMP, room temperature.
chitosan derivative varied depending on the DS and the sodium hydroxide concentration
used in quaternization.
2.4 Acyl Chitosan
Comparing N-alkylation with acylation, the latter is more versatile because it allows the
introduction of hydrophobic moieties at amino, alcohol, or both residues. Moreover, the
introduction of a hydrophobic moiety with an ester linkage allows the action of lipase-like
enzymes, these derivatives being very interesting as biodegradable materials.
N
-Acyl chito-
san derivatives with different purposes have been synthesized as shown in
Table 2.1
[33].
2.4.1
N
-Acyl Chitosan
N
-Acyl derivatives of chitosan can be easily obtained from acyl chlorides and anhydrides
(
cf.
Figure 2.12).
In a general way, acylation reactions carry out frequently in mediums
as aqueous acetic acid/methanol, pyridine, pyridine/chloroform, trichloroacetic acid/
dichloroethane, ethanol/methanol mixture, methanol/formamide, or DMA-LiCl [60]. Due


















Search WWH ::

Custom Search