Biomedical Engineering Reference
In-Depth Information
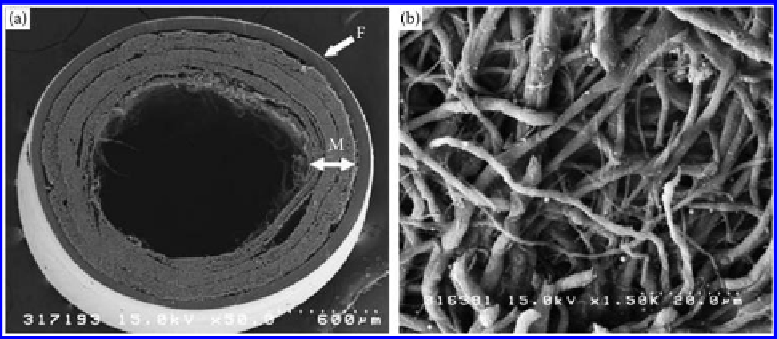
Figure 9.37
(a) SEM micrographs of the bilayered chitosan tube. F, chitosan film; and M, electrospun nano/microfiber mesh.
(b) Enlargement of the electrospun nano/microfiber mesh. The nano/microfiber structure comprises randomly
oriented fibers. 3D pores formed among fibers are interconnected and distributed throughout the structure.
(From Wang, W. et al. 2008.
J Biomed Mater Res
84A: 557-566; Wang, W. et al. 2008.
J Biomed Mater Res
85A: 919-
928. With permission.)
outer layer of chitosan film and an inner layer of electrospun chitosan nonwoven have
been developed (
cf.
Figure 9.37) [194,195]. 3D pores formed between fibers were intercon-
nected and distributed throughout the structure. The porosity improves the exchange of
nutrients and metabolic waste between the scaffold and surrounding tissue. The compres-
sive strength of the bilayered chitosan conduits was significantly greater than that of the
chitosan fiber mesh conduits. The bilayer chitosan-based conduits can improve nerve
regeneration, and the efficiency of nerve regeneration into bilayer chitosan tubes with
immobilized CGGGGGGYIGSR peptide was similar to that of the isograft. Axonal regen-
eration parallel to aligned Schwann cells is reported in injured peripheral and central
nerves
in vivo
. Inducing Schwann cell alignment resulting in oriented axonal growth
while preventing neuroma formation in the peripheral nerve injury is very important
during the process of nerve regeneration. Itoh and coworkers [196] constructed bilayered
chitosan fiber conduits with an inner layer of oriented nanofibers and an outer layer of
randomized nanofibers. Schwann cells aligned in the same direction as a result of secure
adhesion to the oriented fibers, but had no specific orientation on randomized nanofibers.
The oriented chitosan nanofiber mesh tube may be a promising substitute for an autoge-
nous nerve graft.
The degradation of chitosan used to date in nerve regeneration is very slow and poorly
controlled. As a source material of chitosan, chitin has attracted much less research inter-
est mainly because of the difficulty in processing it into the desired shape and also because
of uncertainty of its biological properties, especially nerve cell affinity. Gu and coworkers
[197] developed a two-step procedure to prepare tailored chitin products indirectly starting
from chitosan counterparts. This “chitin” and chitosan materials were equally biocompatible
to cultured Schwann cells. The degradation rate of “chitin” is higher than that of chitosan.
Moreover, the shapes of implanted chitin conduits in nerve defects were completely
changed, turning into a number of opalescent pieces, whereas the shapes of the chitosan
conduits still showed no conspicuous alterations and there was little inflammation at
the implantation spots (
cf.
Figure 9.38)
.
Carboxymethyl chitosan, a dissolvable chitosan
derivative, also possesses many desirable physiochemical and biological features that are
Search WWH ::

Custom Search