Biomedical Engineering Reference
In-Depth Information
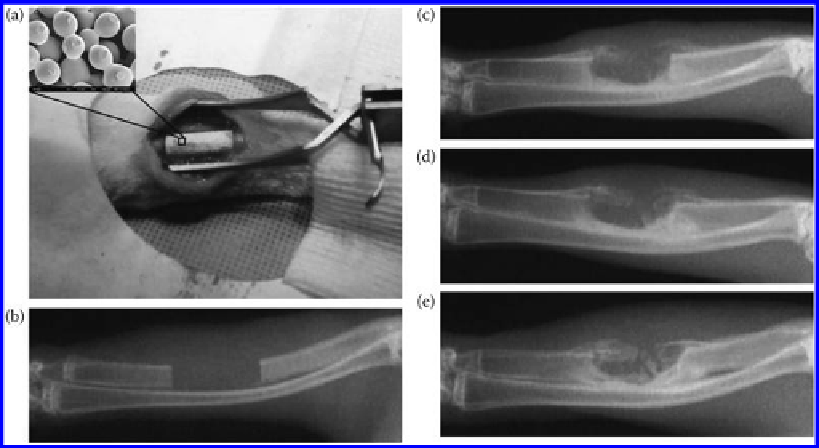
Figure 9.32
(a) The surgical procedure showing that a 15 mm segment of ulna was removed and a sintered microsphere scaf-
fold was implanted into the defect site; (b) a radiograph of the defect immediately after surgery showing the removal
of the segment of ulna and the creation of a segmental defect in the ulna; (c-e) typical radiographs of the defect site
at 4 weeks (c), 8 weeks (d), and 12 weeks (e) post operation in the heparinized chitosan/PLAGA microsphere scaf-
folds. The inset in (a) shows the porous structure of the sintered microsphere scaffold used in the
in vivo
st udy.
when compared with pure chitosan scaffolds. Chesnutt et al. [172] found that chitosan-
HAp scaffolds were also shown to be biocompatible and osteoconductive in a preliminary
critical-sized rat calvarial defect study. After 12 weeks, composite scaffolds were enclosed
by a thin layer of new bone and some small scaffold particles were observed to have
become completely incorporated into the new bone. Moreover, the combination of the
chitosan-HAp scaffolds and osteoblasts can achieve a better repair of bone defect [173].
In order to further mimic the composites and structure of nature, collagen or gelatin also
be combined into chitosan-HAp scaffold. Chitosan-gelatin-HA composite scaffolds were
prepared via blending chitosan-gelatin solution and HA particles. Osteoblasts adhered to
the surface of the composites, performed their functions, and exhibited a good prolifera-
tion in the composite scaffolds, while interlaced ECM networks formed around the cells (
cf.
Figure 9.33)
[174]. The osteoblast-scaffold constructs had good biomineralization effect
after 3 weeks in culture. Hu and coworkers [175] found that chitosan-collagen-HA scaf-
folds have a capability for bone repair. When the composites scaffolds were implanted in
the bone defect site of rabbits, the bone defect was still there at the fourth week, and there
was no new bone formed in the bone defect site. At the eighth week, defect could still be
found in the bone defect location. At the 12th week, the bone defect was repaired com-
pletely (
cf.
Figure 9.34).
However, the HAp particles in this case did not disperse well or
were easily to agglomerate, and even settle, which made it difficult to form a controlled
structure. Meanwhile, the HAp crystal may migrate from the composite because of
the weak interactions between HAp and the chitosan matrix. When cells interact with the
composites, the interfaces between HAp crystals and polymer were destroyed and the
nHA crystals were easily released from the chitosan-based matrix. The composite scaffold
obtained via deposition
in situ
can avoid these disadvantages, because there are strong
Search WWH ::

Custom Search