Biomedical Engineering Reference
In-Depth Information
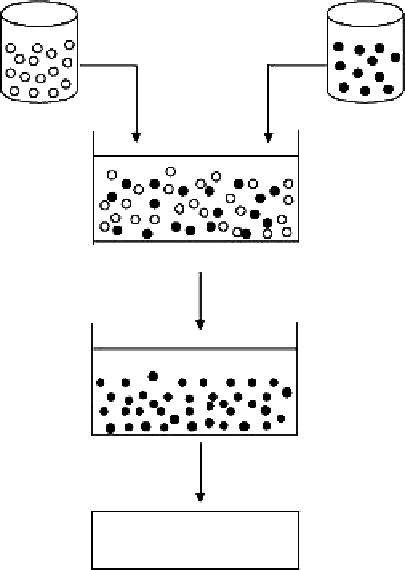
Chitosan emulsion (w/o)
NaOH emulsion (w/o)
High-speed stirring
Solidification of chitosan
Centrifugation and washing
Separation of particles
Figure 7.4
Schematic representation of the preparation of chitosan particulate systems by the emulsion-droplet coales-
cence method.
to give small-sized particles. The method is schematically shown in Figure 7.4 [1].
Gadopentetic acid-loaded CS nanoparticles have been prepared by this method for gado-
linium neutron capture therapy. Particle size depends on the type of CS. When the
deacetylation degree of CS decreases, the particle size increases, but the drug content
decreases. Particles produced using 100% deacetylated CS had the mean particle size of
452 nm with 45% drug loading. Nanoparticles are obtained within the emulsion droplet.
The size of the nanoparticle does not reflect the droplet size. Since gadopentetic acid is a
bivalent anionic compound, it interacts electrostatically with the amino groups of CS,
which would not have occurred if a cross-linking agent, which blocks the free amino
groups of CS, is used. Thus, it is possible to achieve higher gadopentetic acid loading
by using the emulsion-droplet coalescence method compared to the simple emulsion
cross-linking method.
7.2.1.5 Ionic Gelation
In the ionic gelation method, CS is dissolved in an aqueous acidic solution to obtain the
cation of CS. This solution is then added dropwise under constant stirring to polyan-
ionic tripolyphosphate (TPP) solution. Due to the complexation between oppositely
charged species, CS undergoes ionic gelation and precipitates to form spherical particles.
The method is schematically represented in
Figure 7.5
[1]. The use of complexation
between oppositely charged macromolecules to prepare CS microspheres has attracted
Search WWH ::

Custom Search