Biomedical Engineering Reference
In-Depth Information
100
80
60
40
COS-DOX/pluronic
COS-DOX/pluronic + free doxorubicin
GM-COS/pluronic + free doxorubicin
Pluronic + free doxorubicin
20
0
0
5
10
Time (days)
15
20
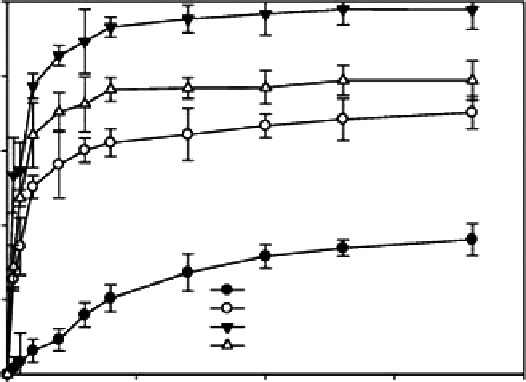
Figure 6.15
In vitro
-release profile of doxorubicin hydrogels at 37°C. Each point is mean ± standard deviation (
n
= 3).
(COS-DOX represented doxorubicin-chitosan conjugates). (From Cho, Y. I. et al. 2009.
Eur J Pharm Biopharm
73:
59-65. With permission.)
6.5.4 Hydrophobic Drug loading into Hydrogels
Due to their inherent hydrophilic nature, hydrogels have been effectively used to deliver
hydrophilic small-molecule drugs that have high solubility in both the hydrophilic hydro-
gel matrix and the aqueous solvent swelling the hydrogel. However, with the development
of pharmaceutics and life science, some hydrophobic drugs are becoming increasingly
important clinically to cure disease and regenerate tissue. The issue of loading and deliv-
ering poorly soluble drugs proves to be a difficult challenge, because hydrophobic drugs
are sparingly soluble in both the aqueous and the hydrogel phases.
Previous strategies of loading hydrophobic drugs include the multiple emulsion tech-
nique. In this method, the drug is dissolved in a suitable solvent and then emulsified in
chitosan aqueous solution in order to form an oil-in-water (o/w) emulsion. Also a surfactant
stabilizing the emulsion is added. This o/w emulsion can be further emulsified into liquid
paraffin to obtain multiple emulsions. By using a suitable cross-linking agent, drugs are
loaded into the chitosan matrix. Organic solvents may make it difficult to completely remove
them. In addition, the strategies mentioned above can also be employed to improve hydro-
phobic drug loading into hydrogels. However, sometimes modification of the drugs and
chemical reaction may lead to weakening the activity and therapeutic efficiency of the drugs.
Compatibility between hydrophobic drugs and the hydrophilic network can be accom-
plished by introducing hydrophobic domains directly into otherwise hydrophilic hydrogel
networks instead. In this way, the loading of hydrophobic drugs is improved significantly.
The most common approach for generating hydrophobic domains within the bulk
hydrogel is by attaching small hydrophobic moieties to the polymer before gelation. Martin
et al. [115] prepared hydrophobic pendants containing chitosan (palmitoyl glycol chitosan)
hydrogels by freeze-drying an aqueous dispersion of the polymer in the presence of a
hydrophobic drug denbufylline. Results showed that the sustained delivery of denbufylline
Search WWH ::

Custom Search