Biomedical Engineering Reference
In-Depth Information
were introduced into chitosan. After UV irradiation, the azide is converted into a reactive
nitrene group that binds chitosan's free amino groups, causing gelation within 60 s. The
acrylate group is another common photosensitive group and is often used in photopoly-
merization [75,76]. Photopolymerizable chitosan derivatives have been prepared previ-
ously through methacrylation using reaction aldehyde intermediates [77]. However, this
photopolymerizable chitosan derivative is only soluble at acidic pH, whereas solubility at
physiological pH values is required for the synthesis of useful biocompatible hydrogels for
biological applications. Thus, a useful chitosan-based photopolymerizable precursor
should be solubility in aqueous solution at physiological pH. Gao et al. [76] prepared such
a water-soluble (methacryloyloxy)ethyl carboxyethyl chitosan as a photopolymerizable
prepolymer through Michael addition reaction between chitosan and ethylene glycol acry-
late methacrylate. By blending prepolymer with D-2959 photoinitiator in solution, hydro-
gels were created under UV irradiation.
Ease of formation, high gelation speed, and so on make photopolymerization a promis-
ing way to obtain
in situ
forming hydrogel. However, toxicity of photoinitiator and expo-
sure to UV irradiation may result in protein denaturation when protein drug was loaded
or may do harm to neighboring cells and tissues, which should be taken into account when
choosing the method.
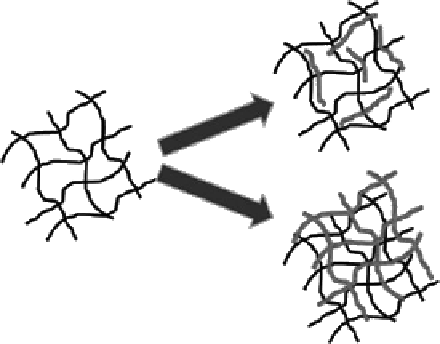
On the other hand, as is well known, the interpenetrating network (IPN) technique can
be used to improve the properties of chitosan-based gels [51,78]. A cross-linked chitosan
network can be allowed to swell in an aqueous solution of polymer monomers. These
monomers are then polymerized, forming a physically entangled polymer mesh called
IPN. When the second polymer is linear (without being cross-linked), a semi-IPN results.
When the second polymer is also cross-linked, a full-IPN is formed (
cf
. Figure 6.11).
Chitosan-based IPNs can also be formed as follows: Take IPNs of PNIPAAm and chitosan
for example [78]; their IPNs were prepared by free radical polymerization and cross-link-
ing of NIPAM with bis(acrylamide) in chitosan solutions and subsequent immersion in
glutaraldehyde solutions to post-cross-link the chitosan.
The IPN technique allows for the specific selection of polymers that can complement the
deficiencies of one another. For instance, a hydrophilic polymer can be chosen to enhance
the structural characteristics of the hydrogel, while a biocompatible polymer may limit the
Semi-IPN
Original
hydrogel
+ monomer,
initiator
+ monomer,
cross-linker,
initiator
Full-IPN
Figure 6.11
Formation and structure of semi- and full-IPNs. (From Hoare, T. R. and Kohane, D. S. 2008.
Polymer
49: 1993-
2007. With permission.)
Search WWH ::

Custom Search