Biomedical Engineering Reference
In-Depth Information
CH
2
OH
CH
2
OH
O
O
NaOH, CS
2
O
O
OH
OH
*
*
*
*
NH
n
NH
2
n
C
S
S
Na
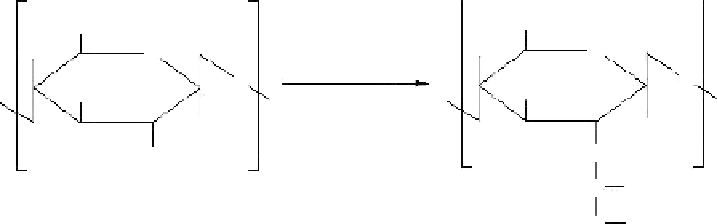
Figure 1.12
Transformation of chitosan to xanthate.
Etherification of chitin includes the following steps: making frozen basified chitin by
using chitin and concentrated alkali, directly dispersing it in alkyl halide, slowly stirring
at 12-14°C for 24 h, neutralizing by using dilute acetic acid, filtering and drying deposits
in air, washing them with ethanol, water, and ethanol in sequence, dehydrating with
acetone, and drying the product. The alkyl halide is 10 times the residues in mol. The
substitution degree is unrelated to the amount of alkyl halide. Generally, substitution
degrees are low. Some believe that it is because of temperature, whereas others think it
is because of the tight structure of chitin. Actually, the main cause is the low activity of
alkyl halides. The substitution degree can be increased only by increasing the reactivity.
Primary reactions of alkyl halides and chitosan are N-alkylation followed by O-alkylation
(etherified chitosan).
Chitosan and dimethyl sulfate form methyl ether in alkali medium by the following
steps [69]: dissolving 38 g of chitosan in 1000 mL of 1 mol/L HCl, slowly adding 500 g of
granular NaOH in the solution, stirring until the mixture becomes a paste, adding 500 mL
of water in the paste, stirring, adding 200 mL of cold dimethyl sulfate to the paste in 1 h,
stirring for 8 h, carefully adding 40 g of NaOH and 40 mL of dimethyl sulfate to the mix-
ture in baths, stirring for 48 h, neutralizing with concentrated hydrochloric acid, dialyzing
by using water for 4 h, concentrating the liquid to the smallest volume, and drying by
freezing to form 36 g of chitosan methyl ether whose substitution degree is 29%. Most
substituted groups are hydroxyls, which are turned into ether. A few aminos are also
substituted to form
N
-methyl chitosan.
Site control of etherification is theoretically significant. Chitin is a macromolecule with
complex structure, high crystalline degree, and large molecular weight. C6 hydroxyls and
C3 hydroxyls are alike despite their different levels of activity. In particular, neither of
them can react in homogeneous phase. So it is extremely complicated to increase the
substitution degree, not to mention control of the reaction site. Just a few etherified chitins
or acylated chitins with substitution degrees of 2 (two hydroxyls are completely substi-
tuted) have been reported so far. Jiang [70] was successful in directly changing chitin into
fully benzylated chitin by liquid-solid phase transfer catalysis, and conveniently prepared
6-
O
-benzyl chitin and 3,6-
O
-di-benzyl chitin by controlling the amount of NaOH.
Both chitin and chitosan are available for cyanoethylation with acrylonitrile in the
alkali condition, which forms
O
-cyanomethyl ether and causes many side reactions.
One side reaction is hydrolysis of the cyano of cyanomethyl ether by alkali, forming
O
-propionamido chitin and sodium
O
-carboxylethyl chitin. The cyanoethyl etherification
Search WWH ::

Custom Search