Biomedical Engineering Reference
In-Depth Information
Increasing
temperature
Decreasing
temperature
Chitosan
Pluronic
Water
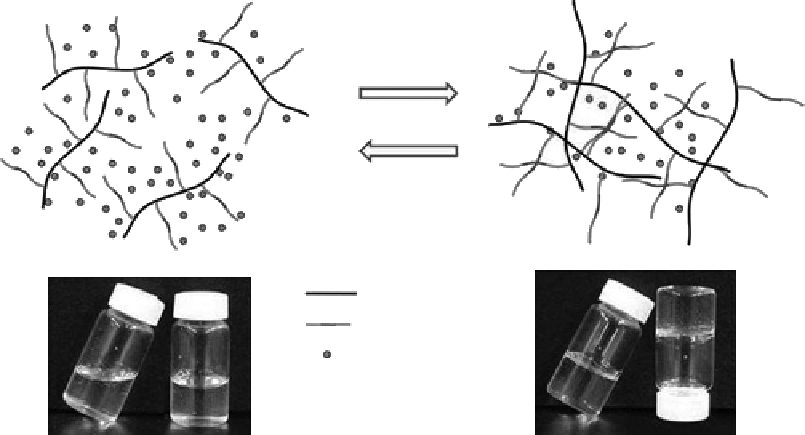
Figure 5.18
Hydrogel formation of the thermosensitive CP hydrogel in aqueous solution via the hydrophobic interaction of
a PPO group in Pluronic and the dehydrated chitosan chain.
dramatically at what is known as the LCST. Experimentally, the LCST of PNIPAAm is in
the range of 30-35°C [59]. At a temperature below its LCST, linear PNIPAAm is water sol-
uble; however, at an LCST temperature or higher, the hydrogen bond interactions between
PNIPAAm and water become weak and water would be released. PNIPAAm would
undergo a coil-to-globule transition and become insoluble. PNIPAAm can be combined
into chitosan-based hydrogels and endow the chitosan-based hydrogels excellent thermo-
sensitivity. Many studies concern chitosan/PNIPAAm hydrogels.
Chitosan/PNIPAAm semi-IPN or IPN is one of the most common thermosensitive
hydrogels. Lee and Chen [60] examined chitosan/PNIPAAm semi-IPN and glutaralde-
hyde-cross-linked IPN hydrogels and found that the swelling ratios of IPN gels are lower
than those of semi-IPN gels, the reason being that the addition of cross-linked chitosan to
the PNIPAAm hydrogel makes the network structure of the gel denser and more hydro-
phobic. The PNIPAAm/chitosan semi-IPN and IPN hydrogels de-swell more quickly at
their gel transition temperatures (
cf.
Figure 5.19)
.
Gel transition temperature for the present
gels is not affected by the addition of the chitosan component in the gels. That is to say, the
LCST of chitosan/PNIPAAm depends on the PNIPAAm. In the chitosan/PPNIPAAm IPN
and the semi-IPN system, chitosan chains and the PNIPAAm network give a relatively
independent polymer system, in which each retained its own properties [61]. For the same
reason, the LCST of the carboxymethyl chitosan/PNIPAAM semi-IPN is also ca. 30-35°C.
In addition, the swelling-deswelling behaviors of the carboxymethyl chitosan/PNIPAAM
semi-IPN hydrogel have good reversibility, and this process may be repeated many times,
with better reproduction [62]. However, incorporation of PEG can improve the LCST,
because PEG would weaken the interactions between PNIPAAm and chitosan, which
leave PNIPAAm more opportunity to interact with water molecules. As a result, more heat
would be required to break the hydrogen bonds. In addition, the PEG-incorporated
chitosan/PNIPAAm would be more hydrophilic, and such an increase in hydrophilicity in
Search WWH ::

Custom Search