Biomedical Engineering Reference
In-Depth Information
hydrophobic polymer segments begin to associate with each other, and hydrogels result.
These polymer solutions exhibit a separation from solution and solidification above a
certain temperature defined as the lower critical solution temperature (LCST). Above the
LCST, they turn into a gel, becoming extremely hydrophobic and insoluble [28]. In gen-
eral, as the polymer chain contains more hydrophobic constituents, LCST becomes lower.
From a thermodynamic point of view, thermoreversible behavior in a polymer system can
generally be regarded as a change in the driving force in response to temperature varia-
tion. From the molecular structure point of view, the thermosensitive hydrogels usually
display a change in hydrophobicity or efficiency of hydrogen bonding when temperature
increases.
Some chitosan-based hydrogels have thermosensitivity. As clinically applicable hydro-
gels, thermosensitive chitosan-based hydrogels preferably assume a liquid form below
body temperature and gel readily at body temperature. In addition, their swelling behav-
iors could change under temperature stimuli. Chitosan possesses hydrophobic (-CH
3
) and
hydrogen bonding favoring groups (-OH, -NH, and -C=O). However, intermolecular
interactions of pure chitosan change little under temperature stimulation due to its high
hydrophobicity. It should provide a favorable environment to form a gel structure under
temperature stimulation through the following mechanisms: (1) Incorporating some func-
tional groups to reduce hydrogen bond interactions between chitosan and water by a
screening effect at low temperature and to enhance the chitosan-chitosan interactions
over those of chitosan-water via the hydrophobic effect at high temperature and (2) intro-
ducing some amphiphilic thermosensitive polymer containing hydrophilic and hydropho-
bic segments. Therefore, some functional group or polymer is introduced into the chitosan
network via grafting, block or blending. These functional groups or polymers can modu-
late the hydrophilic-hydrophobic balance via temperature change, and endow the chito-
san with thermosensitivity (
cf.
Figure 5.12) [29]. If the chitosan chains in hydrogels are not
covalently cross-linked in this system, thermosensitive hydrogels can undergo sol-gel
phase transitions, instead of swelling-shrinking transitions. Moreover, most of these ther-
mosensitive chitosan-based hydrogels also have pH sensitivity due to the presence of
amino groups. If the chitosan-based thermosensitive hydrogels can be turned into liquid
at room temperature, undergoing gelation when in contact with physiological fluids, they
will play a very important role in the applications of drug release, cell encapsulation, and
tissue engineering.
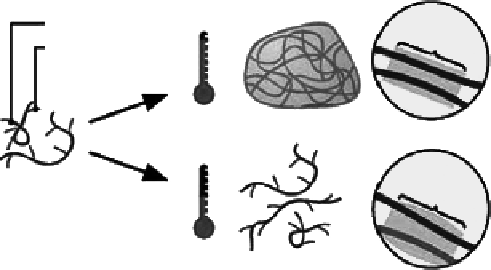
Chitosan
Hydrophilic groups
Hydrophobic
interactions
37°
Hydrogel formation
Hydrophobic-
hydrophilic
repuision
20°
Polymer dissolution
Figure 5.12
Schematic representation of thermoreversible networks of chitosan graft copolymer resulting in semisolid gel
at body temperature and liquid below room temperature.
Search WWH ::

Custom Search