Biomedical Engineering Reference
In-Depth Information
Cross-linked chitosan/poly(oxypropylene glycol) semi-IPN hydrogels based on the com-
plex formed by physical and chemical cross-linking, for example, imine bonds and interac-
tions between macromolecular chains including hydrogen bonding, display pH sensitivity.
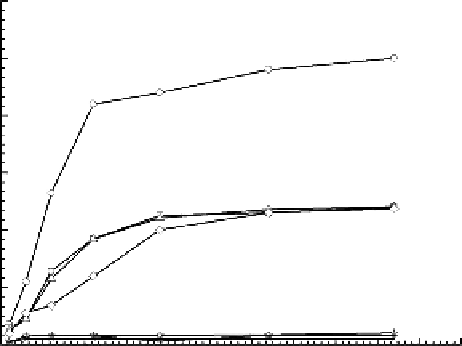
The rate of swelling is highly pH dependent and can be divided into three parts (
cf.
Figure
5.8) [19]: (1) When pH > 6, the swelling degree of the semi-IPN is very limited, because the
inherent hydrophobicity of the hydrogels dominates. The content of bonding water is very
low, almost approaching zero [20]. (2) When pH ranges from 2 to 4.84, the semi-IPN rapidly
swells due to the protonation of amino groups. (3) When pH = 1, the swelling and swelling
rate of the semi-IPN is prior to that in other pHs because of the highest protonation degree
of amino groups in the gels. In acid medium, the composition of the swelling medium has
a complex effect on the state of the water in the hydrogel (
cf.
Figure 5.9).
Moreover, the
swelling behaviors are also relative to the composition of semi-IPN hydrogels. Compared
with pure chitosan cross-linking hydrogels, the semi-IPN has a lower sorption rate and
exhibits low swelling degree because poly(oxypropylene glycol) provides cross-linking in
the semi-IPN. Meanwhile, the swelling reversibility of pure chitosan hydrogels is low
when its medium pH changes interval, but the semi-IPN can maintain excellent swelling
reversibility (
cf.
Figure 5.10)
[21]. That is to say, poly(oxypropylene glycol) within the semi-
IPN not only enhances the flexibility of the semi-IPN, but also influences the swelling
behaviors of the semi-IPN via macromolecular interactions. The poly(oxypropylene glycol)-
containing hydrophobic moieties offer more free volume to water as a definite water con-
tent and restrict its mobility less; therefore, the content of bond water within the semi-IPN
hydrogels is higher than that within pure cross-linking chitosan hydrogels [22].
Some hydrophilic polymers, such as poly(ethylene glycol) (PEG), poly(vinyl alcohol)
(PVA), and poly(vinylpyrrolidone), are blended with chitosan to obtain pH-sensitive
chitosan-based semi-IPN hydrogels. There are several types of hydrogen bonds in this
semi-IPN; some are intramolecular hydrogen bonds and others are intermolecular hydro-
gen bonds. The hydrogels swell the most in acidic medium compared to neutral or basic
12
10
8
6
4
2
0
0
5 0 5 0 5 0 5 0 5 0 5
Time (min)
Figure 5.8
Swelling kinetics of chitosan/poly(oxypropylene glycol) semi-IPN in different pH solutions at 37°C and ionic
strength
I
= 0.1; (O) pH = 1, (
□
) pH = 2, (Δ) pH = 3.19, (◊) pH = 4.84, (
☆
) pH = 6, (+) pH = 7, (×) pH = 8.99, and (
*
)
pH = 12.
Search WWH ::

Custom Search