Biomedical Engineering Reference
In-Depth Information
H
2
O
(1)
(2)
Chitosan chain
ISP
H
2
O
H
2
O
2
O
δ
-
δ
-
Poly(ethylene glycol) methyl ether chain
Phthalimidogroup
Alkyl amine molecule
Stearic acid molecule
δ
-
+
ISP
δ
-
H
2
O: Water
DMF: Dimethylformamide
ISP: Iso-propanol
δ
-
H
2
O
δ
-
δ
-
δ
-
H
2
O
+
-
δ
-
ISP
δ
-
δ
-
+
δ
-
H
2
O
(a)
H
2
O
ISP
δ
-
δ
-
δ
-
δ
-
ISP
δ
-
ISP
ISP
δ
-
δ
-
ISP
H
2
O
δ
-
δ
-
δ
-
+
δ
-
H
2
O
δ
-
ISP
ISP
δ
-
H
2
O
H
2
O
δ
-
δ
-
δ
-
ISP
ISP
(1)
(2)
H
2
O
δ
-
H
2
O
H
2
O
H
2
O
δ
-
δ
-
ISP
δ
-
δ
-
-
-
δ
-
ISP
δ
-
ISP
ISP
δ
-
H
2
O
δ
-
δ
-
H
2
O
δ
-
-
ISP
δ
-
δ
-
δ
-
δ
-
δ
-
-
H
2
O
δ
-
H
2
O
δ
-
(b)
ISP
δ
-
ISP
δ
-
H
2
O
δ
-
ISP
δ
-
-
H
2
O
δ
-
ISP
δ
-
H
2
O
H
2
O
-
-
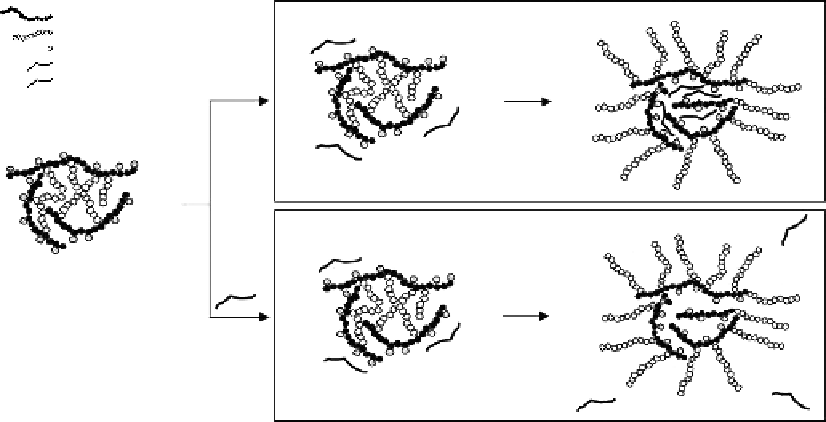
Figure 4.18
Formation mechanisms of
N
-phthaloylchitosan grafted mPEG nanoparticles via the heterogeneous system.
(From Opanasopit, P. et al. 2007.
Colloid Surf B
60: 117-124; Yoksan, R. and Chirachanchai, S. 2008.
Bioorg Med
Chem
16: 2687-2696. With permission.)
efficiency and stability of drug-loaded micelles. And the DD of chitosan is a key factor in
controlling the yield, stability of the drug-loaded micelles, and drug release behavior. As
the DD increases, the stability of CPT-loaded micelles increases, and a sustained release is
obtained at high DD [207]. Furthermore, the internalization of the drug in the micelles
notably hinders the hydrolytic opening of the lactone ring in the physiological environ-
ment (PBS buffer, pH 7.4) and in human serum albumin (HSA) [206,208].
N
-Phthaloyl-carboxymethylchitosan with suitable balance of the attractive and repul-
sive forces could self-organize in a selective and repulsive to form the multilamellar vesi-
cles derivative. It could be self-assembled to form various morphologies of crew-cut
aggregates including vesicles, vesicle encapsulating vesicles, onion-like vesicles, and large
compound micelles in the mixture system. The process can be controlled by adjusting the
concentration of
N-
phthaloyl-carboxymethylchitosan and the ratio of
N
,
N
-DMF in the
mixture solution [209]. The nanovesicles may be used as time release devices. For choles-
terol-modified
O
-carboxymethyl chitosan (
O-
CM-chitosan), the formation of the self-
aggregated nanoparticles is due to the hydrophobic interactions of cholesterol moieties in
aqueous media, and the negatively charged carboxymethyl groups also play an important
role in the morphology and stability of nanoparticles. The critical assembly concentration
of
O-
CM-chitosan conjugates decreases from 0.03 to 0.006 mg/mL when the DS of choles-
terol moiety increases from 6.9% to 12.5%. This is because the increase of hydrophobicity
makes
O-
CM-chitosan molecules easier to aggregate in water [210]. DS also influences the
interaction between
O-
CM-chitosan self-assembly nanoparticles and bovine serum albu-
min (BSA). The higher-order structure of BSA changes on interaction with
O-
CM-chitosan
self-assembly nanoparticles and its stability against a denaturant such as urea remarkably
improves [211]. The hydrophobic anticancer drug, paclitaxel (PTX), can spontaneously
transfer from the aqueous medium into the hydrophobic cores of
O-
CM-chitosan (with DS
Search WWH ::

Custom Search