Biomedical Engineering Reference
In-Depth Information
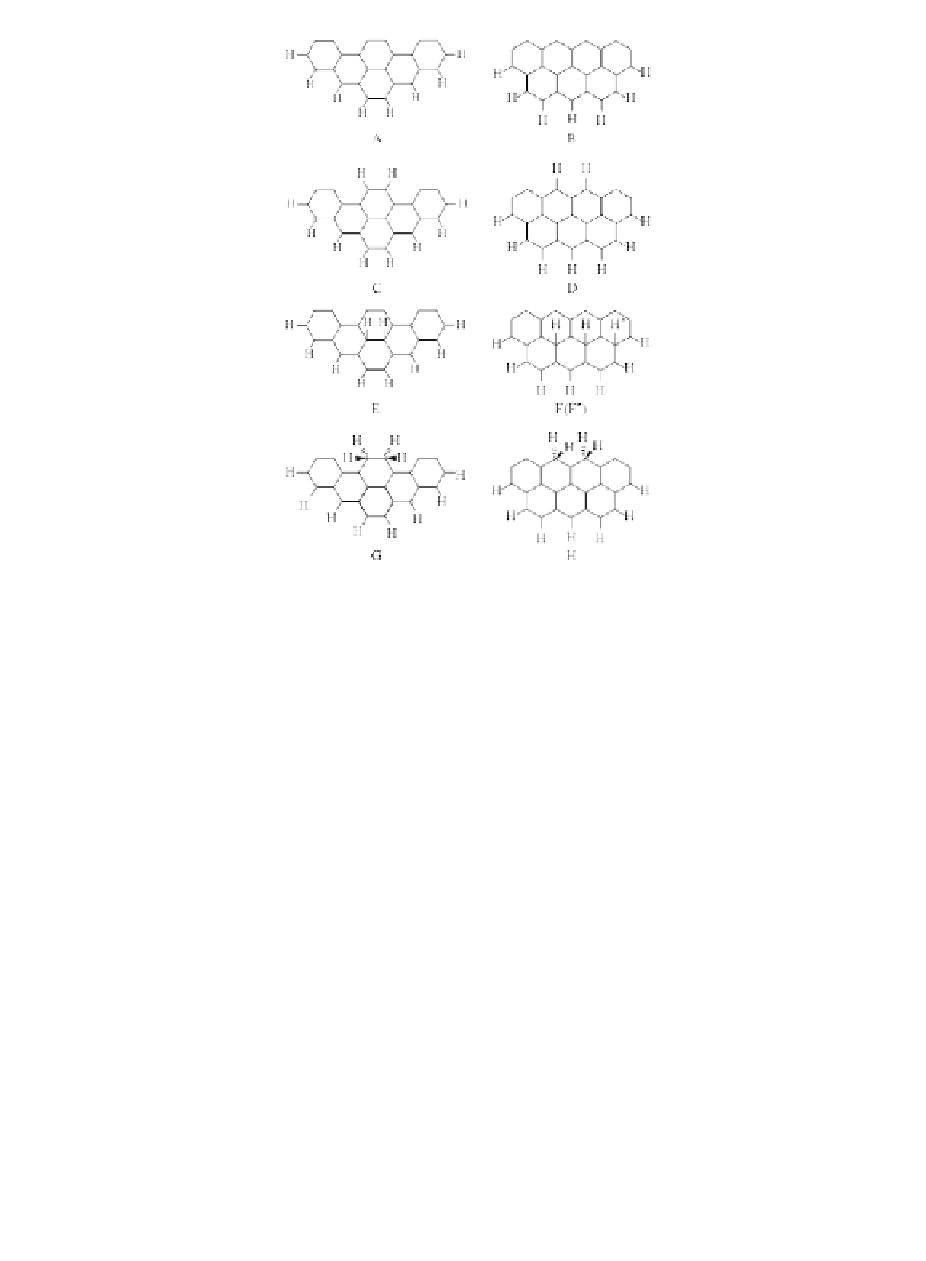
Figure 2.8
Theoretical models of hydrogen atom chemisorptions on
graphite [36].
Using the model described in Refs. [10, 47] for the bulk diffusion
of hydrogen atoms in the graphite lattice accompanied by a reversible
capture on the internal chemisorption centers in graphene layers, we
can write the effective diffusivity (
D
) and the effective difusion-
III
activation enthalpy (
Q
) as
III
1
A
D
,
D
III
(2.8)
III
K
(3)III
(2.9)
D
l
D
Q
Q
-
H
-
H
,
III
(3)III
(3)III
where
is the equilibrium constant for reaction
(2.3) as applied to process III, and
A
≈ const.,
K
III
(3)III
l
l
are the diffusivity and
the diffusion-activation enthalpy, respectively, for hydrogen atoms
in a graphite lattice in absence of chemisorption capture centers or
at maximum (carbohydride) filling of these centers. Knowing the
experimental values of
D
and
Q
Q
and ∆
H
, we can obtain the indirect
III
(3)III
l
−1
value
Q
= 7 ± 4 kJ mol
(H)m by Eq. (2.9).