Biomedical Engineering Reference
In-Depth Information
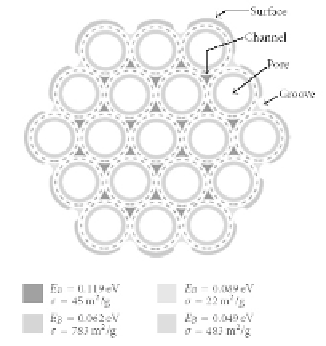
The high capability of gas adsorption into carbon nanostruc-
tures is preparatory for high sensitivity in CNT-based chemical
microsensors. It was demonstrated [81] (see Fig. 9.24) that a
networked bundle of CNTs produces at least four distinct sites in
which the gas molecules can adsorb depending on the characteristic
binding energy: on the external bundle
formed at
the contact between adjacent tubes on outside of the bundle; within
an interior
surface
; in a
groove
pore
of an individual tube; and inside an interstitial
channel
formed at the contact of three tubes in the bundle interior.
Generally, the
site may be activated by chemical modifications
of the CNT bundle with various functionalizing materials like
metallic nanoclusters, polymers, macromolecules, receptors, DNA,
etc. exhibiting the lowest binding energy. This means that this site
is highly activated in the functionalized CNTs upon gas adsorption of
targeted molecules.
surface
Figure 9.24
Schematic structure of a SWCNT bundle showing the
available sites for gas adsorption. Dashed lines indicate the
skeleton of the nanotubes. Binding energies (
E
) and specific
B
surface area (
adsorption on these sites are indicated.
This figure is reprinted and adapted with permission from
Elsevier [81].
s
) for H
2
Polymers are used in chemical sensing due to their reactivity
with gas molecules caused by changes in their physical (e.g., volume
changes upon exposure) and chemical (e.g., change of oxidation
state) properties. They exhibit, particularly the class of the conducting
polymers, very attractive and interesting electronic, optical, magnetic,
mechanical properties and processing advantages.