Biomedical Engineering Reference
In-Depth Information
the SEM image of the PPy matrix, SWCNTs bundles filler with
a diameter of 20 nm, and SWCNT/PPy nanocomposite. This
nanocomposite showed a better sensitivity to NO
gas than both
pure materials of SWCNTs and PPy that was due to the enhanced
conductivity of the composite material by dispersed CNTs. This PPy-
NT nanocomposite sensor exhibited signal degradation under long
time exposure.
2
. [177] fabricated a nanocomposite of MWCNTs
embedded in poly(2,5-dimethylaniline) (PDMA) by oxidative
polymerization. The nanocomposite PDMA-MWCNTs showed
a progressive spontaneous undoping process along the time
associated to the instability of the doping agent, constituted by HCl
vapors, inside the polymeric matrix. The instability of doping process
allowed to fabricate a spontaneous reversible sensor for acid vapors
by setting up a comparative potentiometric circuit and engineering
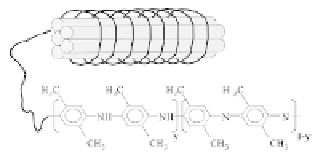
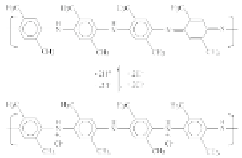
the sensitive element directly onto a circuit board. Figure 9.19 shows
the schematic of PDMA conducting polymer wrapped up around
MWCNTs, and the scheme of doping process of PDMA-MWCNTs
composite.
Bavastrello
et al
(a)
(b)
Figure 9.19
(a) Schematic of PDMA conducting polymer-wrapped
MWCNT film. (b) Scheme of reaction of the doping process of
the PDMA-MWCNT nanocomposite. This figure is reprinted
and adapted with permission from Elsevier [177].
. [178] developed composite thin film of poly-
methylmethacrylate (PMMA) with unmodified MWCNTs or
oxidation-modified MWCNTs (f-CNTs) as nanofillers for gas sensing.
The resistance changes of both nanocomposites were evaluated
upon exposure toward different gases including dichloromethane,
chloroform, acetone, and other volatile organic compounds. Both
CNT/PMMA and f-CNT/PMMA composites showed increasing
resistance upon exposure to these vapors at room temperature. This
Philip
et al