Biomedical Engineering Reference
In-Depth Information
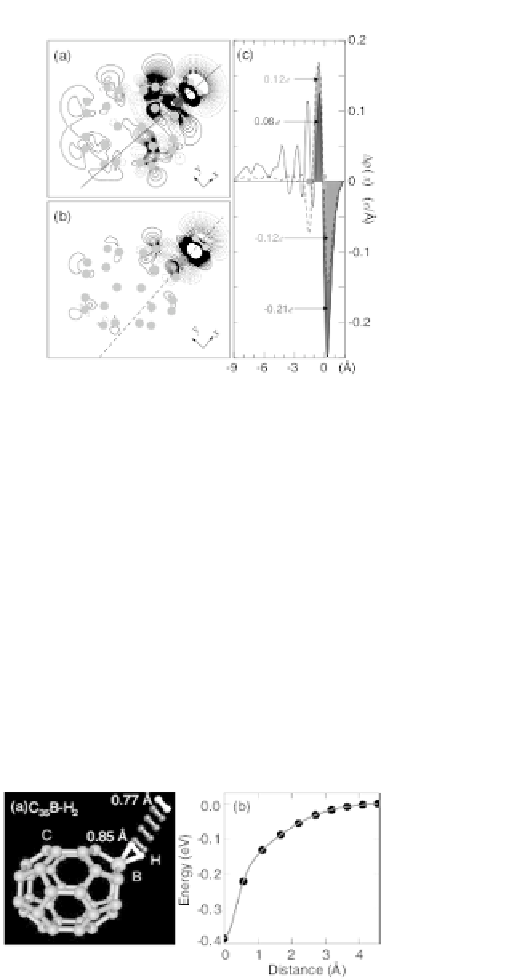
Figure 8.7
Differential planar electron density,
) for B (a) and
Be (b). Solid and dotted contours indicate electron accumulation
and depletion, respectively. Linear
D r
(
x
,
y
D r
(
x
) for B and Be along
the
axis shown in (a) and (b), respectively (c). The positions
of the H
x
and B(Be) are indicated. From Ref. [52]; copyright
2006 by the American Physical Society.
2
to the hydrogen axis (see Fig. 8.7), shows that, in the case of B, only
few electrons are involved in the formation of a three-center bond,
in contrast with the Be case; therefore, the hydrogen adsorption
energy for Be is larger than the one for B. In the previous case, the
authors claimed that nondissociative chemisorption may occur and
thus H
adsorption/desorption cycles can be easily performed by
exceeding the adsorption energy as drawn in Fig. 8.8 for B doped
fullerene. Dissociative hydrogen adsorption has been studied in
2
Figure 8.8
H
B. Ball-and-stick model in which only four
intermediate (darker) H
binding onto C
2
35
positions are shown (a). Total energy
curve as a function of the H
2
distance from its minimum-energy
position. From Ref. [52]; copyright 2006 by the American
Physical Society.
2