Biomedical Engineering Reference
In-Depth Information
H
physical adsorption in CNTs has been mainly studied by
GCMC and molecular dynamics simulations, even using the simple LJ
potentials that have been proven to give realistic results. Molecular
hydrogen can be adsorbed on the external (exohedral) or on the
internal surface (endohedral) of a SWCNT where adsorbed atomic
hydrogen is unstable and only molecular hydrogen can exist [63].
The endohedral storage capacity is limited by steric hindrance
phenomena so that excessive hydrogen storage results in large
repulsive energies and, eventually, in breakdown of the tube wall.
The LJ parameters for carbon-gas interactions have been treated
quite often using the well known Lorentz-Berthelot rules:
2
s s
gg
CC
.
s
e e e
;
.
(8.12)
gC
gC
gg CC
2
s
e
s
e
where
are the LJ gas/gas parameters and are the
LJ carbon/carbon parameters, respectively.
,
, and
,
gg
gg
CC
CC
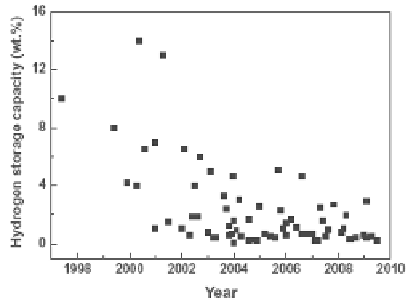
Figure 8.1
CNT hydrogen storage capacity reports vs. the year of
publication (from [68]).
Stan and co-workers [92, 93] have integrated the LJ potential
over an ideal CNT surface for different gaseous species using the
obtained potential for rigid tubes to calculate the uptake of different
gases into various adsorption sites of CNT bundles. Provided that
the adsorbate is small enough (as molecular hydrogen) and that the
CNTs are properly arranged in a honeycomb structure, it comes out
that the hydrogen amount in the interstitial region is not negligible
compared with the amount inside the tubes.