Biomedical Engineering Reference
In-Depth Information
decreasing relative humidity. Beyond this size, the formation of CNTs
in a vacuum (pristine structures) is preferred. Secondly, one should
also consider the overall thermodynamic stability, with respect to
the surrounding environment. At room temperature (
≈ 26 meV)
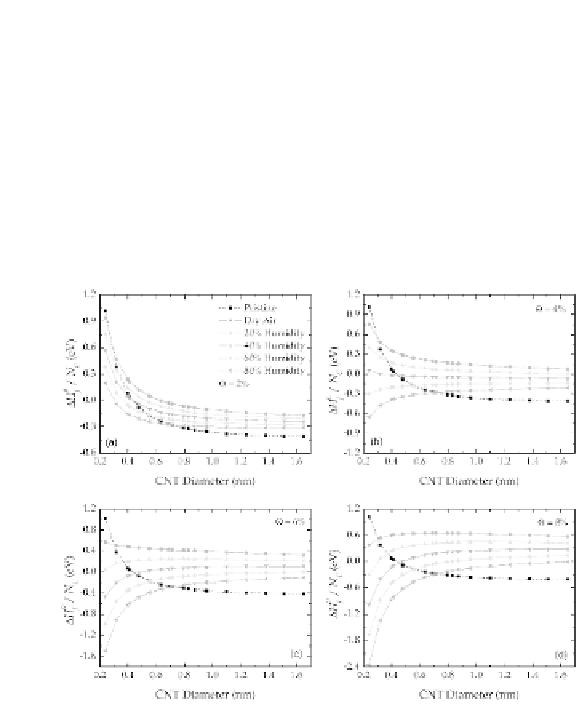
the formation enthalpy at Θ = 2% is exothermic, indicating that
adsorption is thermodynamically favorable, irrespective of humidity.
As the coverage of air increases (Fig. 7.15a-d), formation in drier air
becomes endothermic, and greater degrees of relative humidity are
required to ensure CNT stability.
k
T
B
Figure 7.15
Relative stability of pristine CNTs and equivalent structures
coated with air, with (a) 2%, (b) 4%, (c) 6%, and (d) 8% total
coverage of air, with 0% (dry air), 20%, 40%, 60% and 80%
relative humidity. Reproduced with permission from Ref. [58]
IOP Copyright Publishing, 2009 and Institute of Physics, UK.
This indicates that these air-covered CNTs are unstable with
respect to either disintegration or desorption of certain adsorbates,
depending on the relative bond strengths C-X and C-C. If C-X is a
stronger bond than C-C, then the CNT will break given a suitable
perturbation, and ultimately disintegrate if the concentration of X
is high. Alternatively, if the C-C is a stronger bond than C-X then
desorption of X may be expected rather than disintegration.
Finally, if the relative humidity of the surrounding air is known,
results of this type may be useful for estimating the likely uptake of
air, if we assume that adsorption ceases when Θ is at the point that