Biomedical Engineering Reference
In-Depth Information
any size. When defining Θ, one must also remember that some gas
species adopt a monodentate adsorption configuration (forming a
bond with only one C atom, such as in the case of H and H
O), and in
other cases they may adopt a bond-centered bidentate adsorption
configuration (forming bonds with two neighboring C atoms, such
as in the case of O and N) [55, 58]. This means that
2
N
/
N
= 4 for N
3
sp
X
and O, and
N
/
N
= 2.5
for H
O and H.
3
sp
X
2
Table 7.1
Parameterization of
E
(Θ,
R
,
X
) = [
aR
+
b
] Θ +
cR + d
and
E
(Θ,
X
)
ad
S
=
e
Θ +
f
for
X
= H, O, N and H
O
2
Gas species
a
b
c
d
e
f
H (from H
) -16.16 53.23 -0.10 -0.05 -47.69 2.08
2
O (from O
)
3.65 −1.12 -0.29 1.11 -31.67 2.22
2
N (from N
)
2.79 3.38 -0.39 -2.22 -35.81 2.26
2
H
O (gas)
-4.64 5.44
0.37 -3.08 -74.95 2.08
2
Note:
All values are in units of eV. Reproduced with permission from Ref. [58] IOP
Copyright Publishing, 2009, Institute of Physics, UK.
(a)
(b)
(c)
(d)
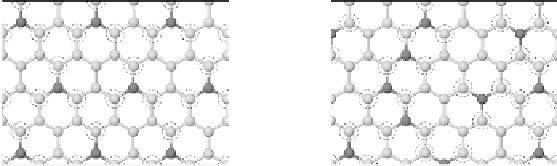
Figure 7.11
Four example adsorption patterns, where all sp
3
-hybridised
C atoms (those with a C-X bond oriented out of the page) are
dark-colored spheres, sp
3
-hybridized C atoms with a dangling
bond are decorated with a circle, and sp
2
-hybridized atoms
are the plain gray spheres. Reproduced with permission from
Ref. [60]; copyright Wiley, 2006.