Biomedical Engineering Reference
In-Depth Information
(Θ > 0), the same procedure must
be repeated for a number of values of Θ. This has been previously
shown to follow a linear trend, and may be fitted to
To obtain an expression for
E
S
E
(Θ,
X
) =
e
Θ +
f
S
for each
) may be determine by
chemisorbing different configurations of gas adsorbates on the
outer wall of a collection of different CNTs (with different chirality
and radii), and for a number of values of Θ. These results may then
be empirically fitted to
X
[58]. Similarly,
E
(Θ,
R
,
X
ad
E
(Θ,
R
,
X
) = (
aR
+
b
)Θ +
cR + d
[55, 58].
ad
Previously reported values for O, N, H and H
O are provided in
Table 7.1, calculated using DFT as described above. In each case, the
energies of the gas molecules were corrected for the (spin-polarized)
energy of the free atomic or molecular species.
2
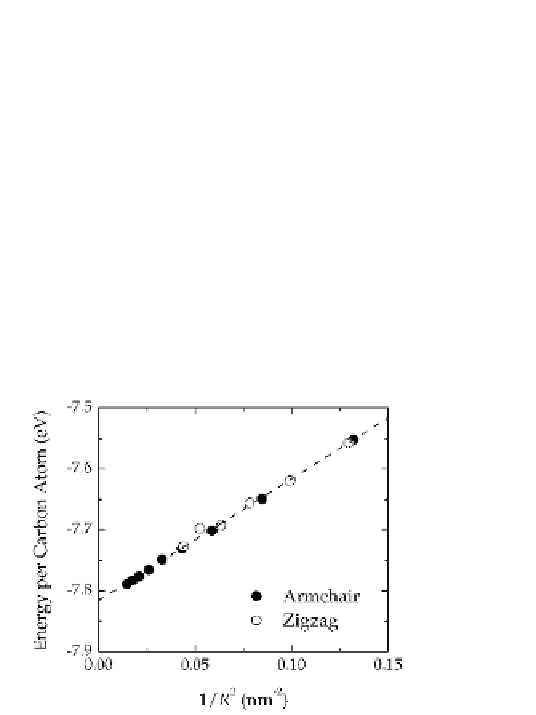
Figure 7.10
Energy per carbon atom calculated for a set of one-dimensional
(infinite) pristine carbon nanotubes. The slope provides the
strain energy
(sp
2
) cohesive energy.
Armchair nanotubes are shown as closed symbols and zigzag
nanotubes are show as open symbols. Reproduced with
permission from Ref. [29]. Cpoyright American Institute of
Physics, 2004.
E
, and intercept the
E
S
2
3
Given the values and expressions for
E
(sp
),
E
(sp
),
E
,
E
(Θ,
R
,
DB
ad
X
), and
E
(Θ,
X
) parameters listed here, and using formation energies
S
0
(
0
D
H
) from experiment or from complementary
calculations, the patterning of the adsorbates and the value of Θ are
all that remain to be defined. These may be thought of as
C
)
and
D
H
(
X
f
f
experimental
parameters
, the manipulation of which facilitates investigation of
the relative stability of different types of coverage of
X
on CNTs of