Biomedical Engineering Reference
In-Depth Information
discussed earlier. We surmise that the energy barrier for H atom
intercalation between graphite sheets is probably less than a barrier
for single H atom adsorption on a graphene face, and so population
of both sides of graphite sheets may be possible.
One implication of these results is that the chemisorption of a
first hydrogen atom actually has the potential of setting of a series
of subsequent adsorption reactions and, upon saturation of the
substrate, of forming the final assembly shown in the left-hand side
of Fig. 5.16.
An independent study with similar conclusions is given in Ref.
[36]. Such a network of fully covered sheets, with its hydrogen-to-
carbon ratio of unity, can hold hydrogen to a commercially practical
storage capacity of 7.7% by weight [35]. This assembly has also been
shown to be the more stable conformation of hydrogen-saturated
graphene, which has a 1:1 C-H stoichiometry [37]. For this material,
an expansion of the graphene lattice parameter by a factor of about
3% has been observed, producing, when fully hydrogenated, C-C
bonds with lengths of 1.54 Å and a structure closer to diamond than
to graphene (also, C-C bond lengths in diamond are 1.54 Å).
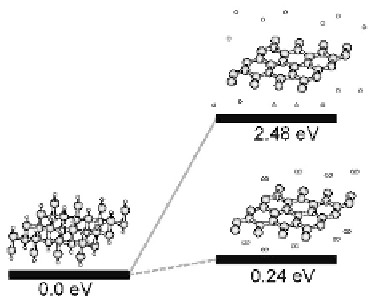
Figure 5.16
Stability of the fully hydrogenated graphene.
This is somewhat in agreement with a recent experimental
work characterizing graphane [38], as local regions with in-plane
expansion have been reported, albeit to a much greater extent.
Along the
plane the hexagonal lattice is maintained. Finally,
the hydrogen-saturated graphene is found to be more stable than
graphene and hydrogen atoms (molecules) by 2.48 eV (0.24 eV), in
terms of chemisorption energy per hydrogen atom (Fig. 5.16) [39].
x
-
y