Biomedical Engineering Reference
In-Depth Information
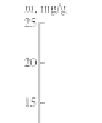
Figure 4.8
Isotherms of adsorption of (1) nitrogen and (2) oxygen at 77 K
on astralene.
4.3
Discussion
Sorption measurements performed for oxygen at various
temperatures and for nitrogen at 77 K on all the adsorbents studied
allow us to estimate adsorption heat, specific surface areas, pore
volumes and sizes, and characteristic adsorption energies.
The adsorption heat gives a direct information on the binding
energy and the nature of adsorption interactions for each adsorbate-
adsorbent pair, and the dependence of the adsorption heat on
surface coverage reveals the energy homogeneity or unhomogeneity
of the adsorbent surface. The adsorption heat is calculated either
directly from adsorption isotherms by determining the isosteric
heats of adsorption,
, or from calorimetric measurements in
the form of differential heats of adsorption,
q
st
q
, at various surface
d
coatings. It should be emphasized that the
values calculated
this way applies only to a definite adsorption value
q
st
Due to the
energy inhomogeneity of the adsorbent surface and to the mutual
interactions of molecules in the adsorbing layer, in general the
adsorption heat vary quite significantly when the adsorbed amount
changes. That is why
m
a
.
q
values should be calculated for each of the
st
sequential
.
It is well known [21] that the isosteric heat of adsorption is
determined by the Clausius-Clapeyron equation
m
values to obtain the trend of
q
dependence on
m
a
st
a
(4.1)
q
- /( ln / ) [ /( - )](ln -ln )
RT
P
T
RT T
T
T
P
P
m
st
1 2
2 1
2
1
a
where
is a heat of
vaporization (condensation), is called the pure heat of adsorption.
q
=
q
+
RT
. The difference
q
-
q
=
q
, where
q
st
d
st
L
0
L