Biomedical Engineering Reference
In-Depth Information
(
) pressure scales. A common feature of these isotherms is that
there is no substantial difference in adsorption values between N
P/P
0
2
and O
for all three adsorbents. This
can be explained taking into account the approximately equal sizes of
N
within a pressure range up to
P
2
0
molecules. These results closely agree with those obtained
by other authors [22-24]. Let us now consider some special features
of the isotherms.
The adsorption isotherms of N
and O
2
2
on activated carbon,
used as a reference adsorbent, are shown in Fig. 4.6. The nitrogen
adsorption isotherm rapidly reaches saturation. This can be
apparently related to complete filling of smaller pores, whereas
larger pores (100-500 nm) remain empty at pressures below
and O
2
2
.
At 77 K, oxygen can be not only adsorbed but also liquefied,
primarily in adsorbent micropores. Therefore, the oxygen sorption
values at pressures much higher than
P
0
should be considered as
a result of simultaneous action of adsorption and gas liquefaction
mechanisms in the micropores, a process of so-called capillary
condensation.
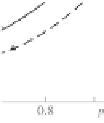
Oxygen liquefaction is especially noticeable for its adsorption
isotherms on fullerene (Fig. 4.7).
Unlike activated carbon and astralene (see Fig. 4.8), oxygen
sorption values on fullerene at pressures higher than
P
0
are larger
than nitrogen sorption values by more than an order of magnitude.
It should be noted that a possible process of oxygen liquefaction
on astralene is observed at gas pressures higher than in the case of
fullerene, which may be associated with the different microporosity
of these materials.
P
0
Figure 4.7
Isotherms of adsorption of (1) nitrogen and (2) oxygen at
77 K on fullerene.